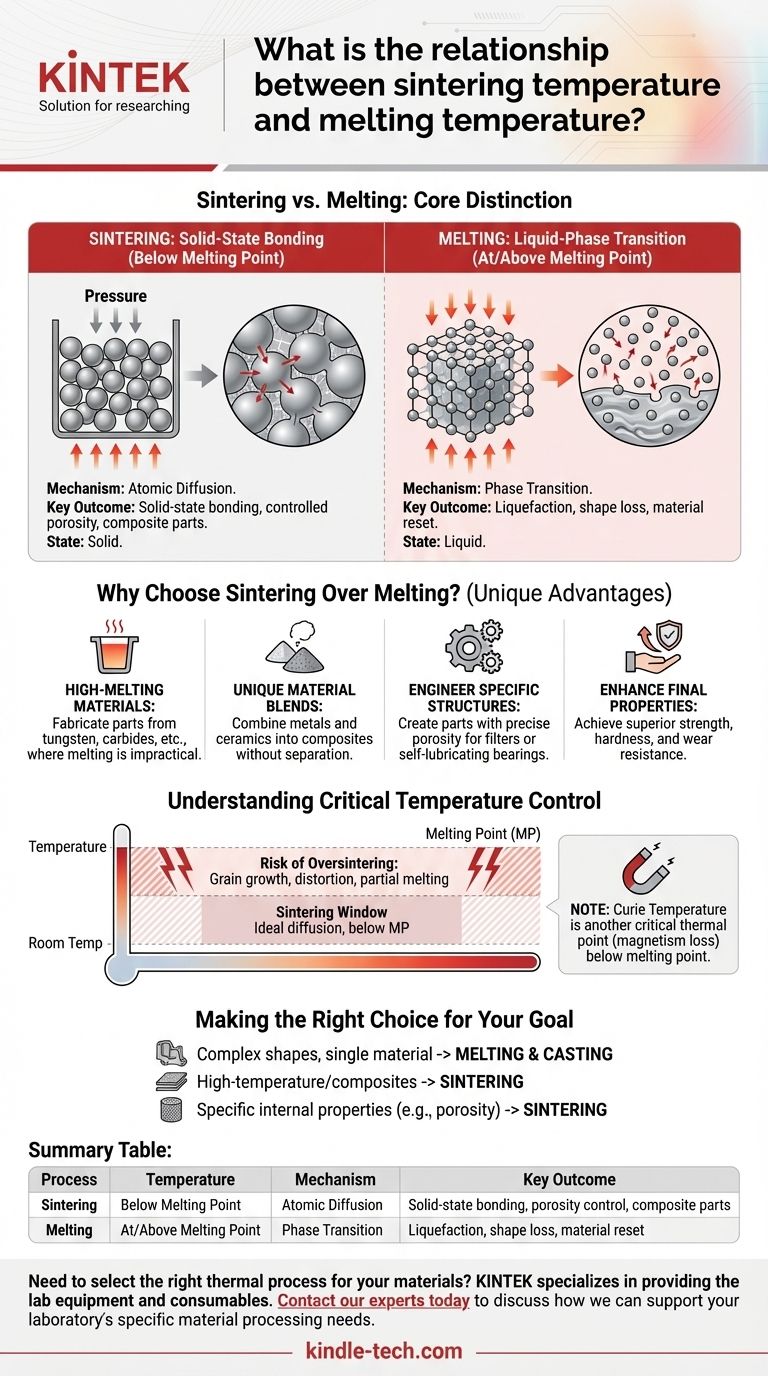
The fundamental relationship between sintering and melting temperature is one of sequence and separation. Sintering is a solid-state process that occurs at a temperature below a material's melting point. In contrast, melting is a phase-change event that happens precisely at or above the melting point, where the material turns from a solid into a liquid.
Sintering and melting are distinct thermal processes defined by their relationship to a material's melting point. Sintering uses heat to bond particles together while they remain solid, whereas melting uses heat to break those bonds entirely, causing liquefaction. This core difference is what enables the unique capabilities of each process.

The Core Distinction: Bonding vs. Liquefying
To grasp the relationship, you must understand that sintering and melting achieve fundamentally different goals at the atomic level. One manipulates a material's structure, while the other completely resets it.
How Sintering Works
Sintering is a process of atomic diffusion. Heat provides energy that allows atoms on the surfaces of individual particles to move and bond with adjacent particles.
This process is often assisted by pressure, which compacts the material and increases the contact points between particles.
The goal is to fuse the particles into a solid mass, increasing its density and strength, all without ever reaching the point of liquefaction.
How Melting Works
Melting is a phase transition. When a material reaches its melting point, the thermal energy is sufficient to break the rigid, crystalline lattice structure holding its atoms in place.
The material changes from a solid to a liquid, losing its original shape and internal particulate structure. Unlike sintering, melting relies solely on thermal energy to achieve this complete state change.
Why Choose Sintering Over Melting?
The deliberate choice to operate below the melting point gives sintering several unique advantages in materials engineering. It is not just a lower-temperature alternative to melting; it is a different tool for a different set of problems.
Fabricating with High-Melting-Point Materials
Sintering enables the creation of parts from materials like tungsten, carbides, or advanced ceramics. Their melting points are so high that melting and casting them would be impractical or prohibitively expensive.
Creating Unique Material Blends
Sintering allows for the combination of materials with vastly different melting points, such as metals and ceramics, into a single composite part. If you were to melt such a mixture, the components would likely separate or react in undesirable ways.
Engineering Specific Internal Structures
The sintering process can be precisely controlled to create parts with a desired level of porosity. This is critical for applications like filters or self-lubricating bearings, a capability that is impossible to achieve through melting and casting.
Enhancing Final Properties
By controlling the temperature, pressure, and time, sintering can produce parts with enhanced properties like superior strength, hardness, and wear resistance compared to the base material powder.
Understanding the Critical Temperature Control
The success of sintering hinges on operating within a specific thermal "window." Deviating from this window can lead to failed parts and negate the benefits of the process.
The Sintering "Window"
For any given material, there is an ideal temperature range for sintering. It must be hot enough to promote significant atomic diffusion but remain safely below the melting point.
The Risk of Oversintering
If the temperature gets too close to the melting point, the process can fail. Excessive heat can cause unwanted grain growth, part distortion, or even partial melting.
This damages the carefully engineered internal structure and compromises the final part's integrity and shape.
A Note on Other Thermal Changes
Melting is not the only critical temperature a material has. For instance, the Curie temperature is the point at which a ferromagnetic material loses its magnetism due to thermal agitation.
This change happens well below the melting point and illustrates a key principle: heat can induce significant changes in material properties without causing a phase change to liquid.
Making the Right Choice for Your Goal
Selecting between these processes requires a clear understanding of your material and your desired outcome. Your decision should be based on the final properties and composition you need to achieve.
- If your primary focus is creating complex shapes from a single, castable material: Melting and casting is often the more direct and economical approach.
- If your primary focus is fabricating parts from high-temperature materials or composites: Sintering is the necessary method, as it avoids the extreme energy costs and material compatibility issues of melting.
- If your primary focus is engineering specific internal properties like porosity or enhanced strength: Sintering provides granular control over the final microstructure that melting cannot achieve.
Understanding this distinction between solid-state bonding and liquid-phase transition empowers you to select the precise thermal process for your material engineering needs.
Summary Table:
| Process | Temperature | Mechanism | Key Outcome |
|---|---|---|---|
| Sintering | Below Melting Point | Atomic Diffusion | Solid-state bonding, porosity control, composite parts |
| Melting | At/Above Melting Point | Phase Transition | Liquefaction, shape loss, material reset |
Need to select the right thermal process for your materials?
KINTEK specializes in providing the precise lab equipment and consumables needed for both sintering and melting applications. Whether you're engineering high-performance composites or working with high-melting-point materials, our solutions ensure accurate temperature control and reliable results.
Contact our experts today to discuss how we can support your laboratory's specific material processing needs and help you achieve superior outcomes.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is a muffle furnace used for? Achieve Pure, High-Temperature Processing
- What is the setting of the muffle furnace? A Step-by-Step Guide to Safe & Accurate Operation
- What is furnace lining? The Engineered System Protecting Your High-Temperature Processes
- What is a muffle furnace used to estimate? A Key Tool for Precise Ash Determination
- What is a muffle furnace in the environment? Achieve Clean, Contaminant-Free Heating



















