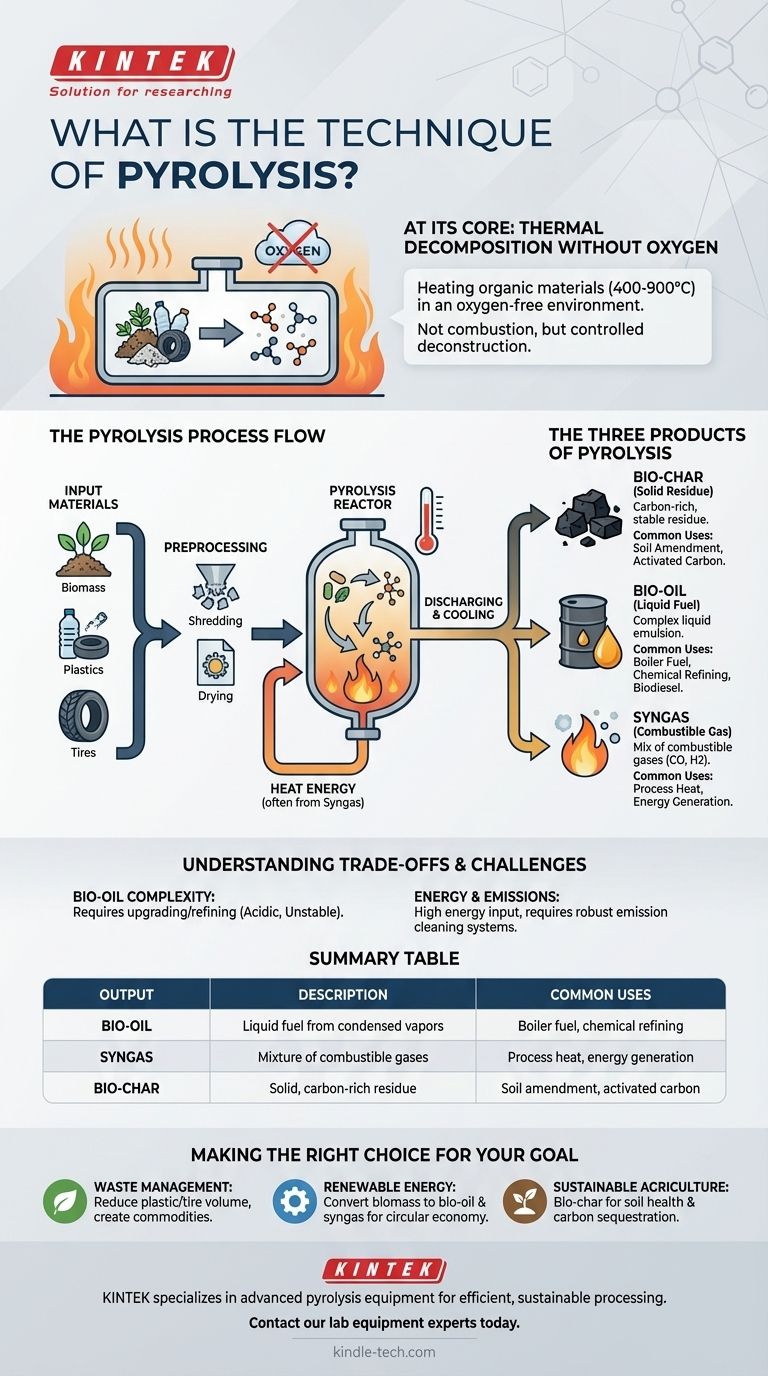
At its core, pyrolysis is a method of thermal decomposition. It involves heating organic materials, such as biomass, plastics, or tires, to very high temperatures in an environment completely devoid of oxygen. Instead of burning, the intense heat breaks down the complex molecular structures of the material into simpler, more valuable substances.
The crucial insight is that pyrolysis is not combustion; it's a controlled deconstruction process. By preventing oxygen from entering the system, it transforms waste or organic matter into three distinct products: a solid char, a liquid oil, and a combustible gas.

How the Pyrolysis Process Works
To understand pyrolysis, it's best to view it as a complete system, from initial preparation to the final output. The entire process is designed around the core principle of controlled heating in an inert atmosphere.
The Core Principle: Heat Without Oxygen
The defining characteristic of pyrolysis is the absence of oxygen. When organic material is heated with oxygen, it combusts (burns), releasing energy, water, and carbon dioxide.
By removing oxygen, the heat energy (typically between 400-900°C) doesn't burn the material. Instead, it cleaves the strong polymer chains in substances like cellulose, lignin, or plastics into smaller, less complex molecules.
The Inputs: What Can Be Processed?
Pyrolysis is versatile and can process a wide range of organic feedstocks. Common inputs include:
- Biomass: Wood, agricultural waste, and other plant matter.
- Plastics: Various types of post-consumer plastic waste.
- Tires: End-of-life vehicle tires.
For the process to be efficient, these materials often require preprocessing, such as shredding, drying, and removing any non-organic contaminants.
The Anatomy of a Pyrolysis Plant
A typical plant is structured around four key operational lines:
- Feeding Line: Prepares and feeds the raw material into the reactor.
- Pyrolysis Line: The sealed, oxygen-free reactor where the material is heated.
- Discharging Line: Safely separates and cools the three end products.
- Emission Cleaning Line: Manages and treats any exhaust to ensure environmental safety.
The Three Products of Pyrolysis
The output of pyrolysis is always a combination of a solid, a liquid, and a gas. The exact ratio depends on the input material and the process temperature.
Bio-char (or Coke)
This is the solid, carbon-rich residue left after the volatile components have been driven off. It's similar to charcoal.
Bio-char is highly stable and can be used as a potent soil amendment to improve fertility and water retention or as a feedstock for producing activated carbon.
Bio-oil (or Pyrolysis Oil)
This liquid is a complex emulsion of water and hundreds of oxygenated organic compounds.
It is a high-density fuel that can be used in some boilers or engines. However, it can also be further refined to produce more conventional biodiesel and other chemicals.
Syngas (or Pyrolysis Gas)
This is a mix of non-condensable combustible gases, primarily carbon monoxide and hydrogen.
A key feature of modern pyrolysis plants is their efficiency; a significant portion of the syngas produced is often redirected back into the system to provide the heat energy required to sustain the reaction.
Understanding the Trade-offs and Challenges
While powerful, pyrolysis is a sophisticated industrial process with inherent complexities that must be managed for successful operation.
The Complexity of Bio-oil
Pyrolysis oil is not a direct substitute for crude oil. It is highly acidic, unstable, and contains a significant amount of water and oxygen.
This means it often requires significant upgrading and refining before it can be used as a transport fuel, adding cost and complexity to the overall process.
Energy and Emission Controls
Reaching and maintaining temperatures of 500°C or higher requires a substantial energy input, though this is often offset by using the syngas produced.
Furthermore, the process handles volatile compounds and requires robust emission cleaning systems to prevent the release of harmful pollutants, representing a critical operational cost.
Making the Right Choice for Your Goal
Pyrolysis is not a single solution but a versatile platform. The value you derive depends entirely on your primary objective.
- If your primary focus is waste management: Pyrolysis is an excellent technology for drastically reducing the volume of plastic or tire waste and converting it into marketable commodities.
- If your primary focus is renewable energy: The process is a viable pathway for converting low-value biomass into bio-oil and syngas, contributing to a circular energy economy.
- If your primary focus is sustainable agriculture: The production of bio-char offers a stable, long-term method for sequestering carbon and improving soil health.
Ultimately, pyrolysis offers a powerful method for unlocking the chemical value stored within organic materials that would otherwise be considered waste.
Summary Table:
| Pyrolysis Output | Description | Common Uses |
|---|---|---|
| Bio-oil | A liquid fuel from condensed vapors | Boiler fuel, chemical refining |
| Syngas | A mixture of combustible gases | Process heat, energy generation |
| Bio-char | A solid, carbon-rich residue | Soil amendment, activated carbon |
Ready to transform waste into valuable resources? KINTEK specializes in advanced pyrolysis equipment and consumables for efficient, sustainable material processing. Whether your goal is waste management, renewable energy production, or sustainable agriculture, our solutions are designed to maximize your output of bio-oil, syngas, and bio-char. Contact our lab equipment experts today to discuss how pyrolysis can benefit your operation!
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the temperature range for biomass pyrolysis? Control Your Output of Biochar, Bio-oil, or Syngas
- What is the bio-oil yield in fast pyrolysis? High Yield, But Quality is Key
- Is plastic pyrolysis eco friendly? A Deep Dive into the Environmental Trade-offs
- What biomass is used to make biochar? The Ultimate Guide to Choosing the Right Feedstock
- What is the difference between a kiln and a calciner? Understand the Key Distinctions in Thermal Processing
- What role does a high-temperature rotary kiln play in the production of cement clinker? Mastering Sintering Efficiency
- What is a calcining kiln? A Guide to Industrial Thermal Processing
- What is the energy efficiency of biomass? Understanding the 20-40% Range for Power Generation



















