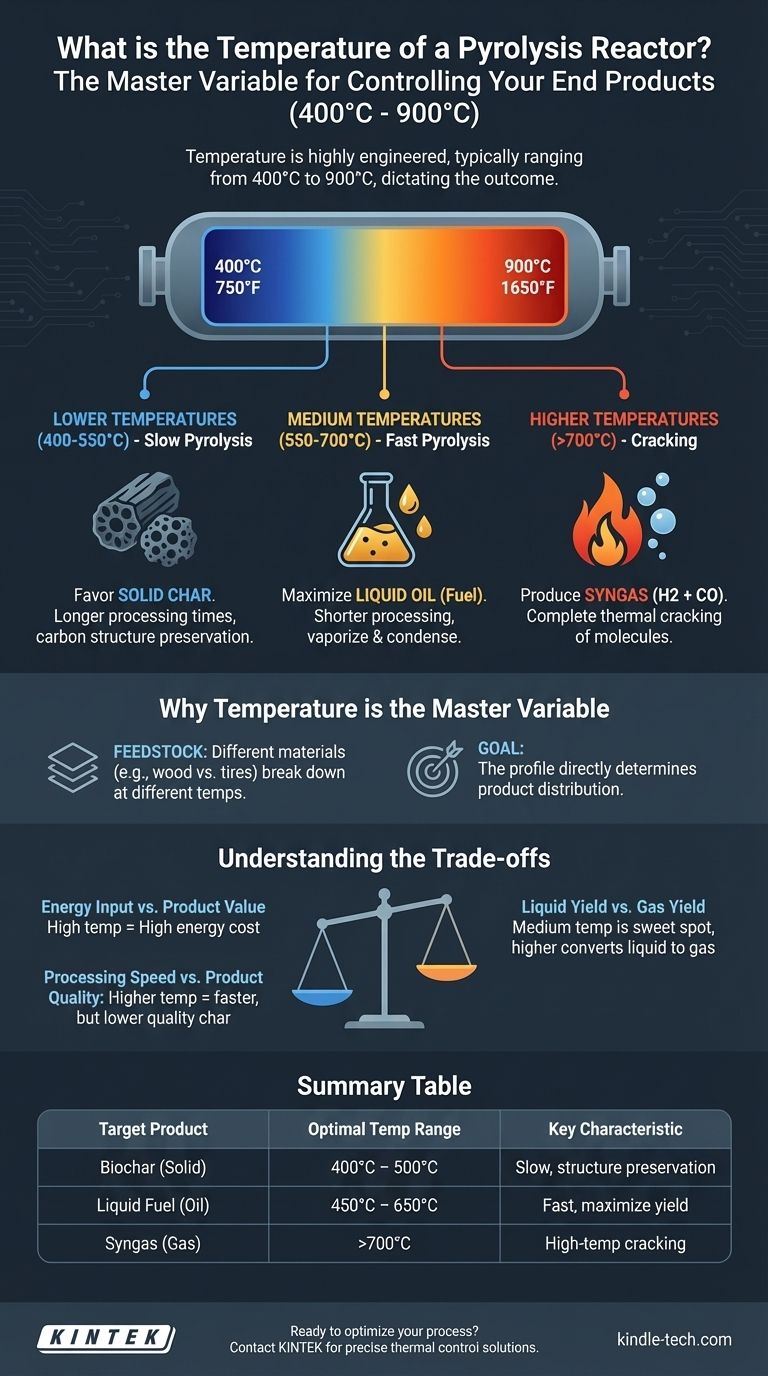
In practice, a pyrolysis reactor does not have one single temperature. It operates within a wide range, typically between 400°C and 900°C (750°F and 1650°F). The precise temperature is the most critical control parameter, as it is deliberately adjusted based on the input material and the desired end products.
The core takeaway is that temperature in a pyrolysis reactor is not a fixed property of the equipment itself. Instead, it is a highly engineered variable that dictates whether you produce more liquid fuel, combustible gas, or solid char from your feedstock.

Why Temperature Is the Master Variable
The goal of pyrolysis is the thermal decomposition of a material in the absence of oxygen. Temperature is the primary lever an operator has to control the outcome of this decomposition, directly influencing both the speed of the reaction and the nature of the products.
The Role of Feedstock
Different materials break down at different temperatures. Lignocellulosic biomass like wood might begin to decompose effectively at 400°C, while more resilient materials like certain plastics or tires may require higher temperatures to break their chemical bonds efficiently.
The Goal: Defining Your End Products
The temperature profile directly determines the final product distribution. A small change in temperature can significantly shift the output from liquid-heavy to gas-heavy.
As a general rule:
- Lower temperatures (e.g., 400-550°C) with longer processing times favor the production of solid char.
- Medium temperatures (e.g., 550-700°C) with shorter processing times are often optimized to maximize liquid oil.
- Higher temperatures (e.g., >700°C) favor the production of syngas (a mix of hydrogen and carbon monoxide) by "cracking" the larger molecules into permanent gases.
How Reactors Are Controlled
A pyrolysis reactor is fundamentally a closed system that operates based on thermodynamic principles. It relies on an external heat supply to bring the feedstock up to the target temperature and maintain it. Designs like a batch reactor are sealed vessels that ensure a controlled, oxygen-free environment, allowing for stable and precise energy application.
Understanding the Trade-offs
Choosing a temperature is an exercise in balancing competing objectives. There is no single "best" temperature, only the optimal temperature for a specific goal, which always involves trade-offs.
Energy Input vs. Product Value
Achieving and maintaining higher temperatures requires a significant energy input. This operational cost must be justified by the market value of the end products. Generating low-value syngas at very high temperatures might only be economical if that gas can be used to power the process itself.
Liquid Yield vs. Gas Yield
While medium temperatures are the "sweet spot" for liquid fuels, pushing the temperature higher begins to convert those valuable liquid vapors into non-condensable gases. Operators must carefully balance temperature to maximize liquid yield without "over-cracking" the molecules into less valuable gas.
Processing Speed vs. Product Quality
Higher temperatures lead to faster reactions, increasing throughput. However, for some products like biochar, a slower, lower-temperature process is essential for creating the desired porous structure and chemical stability. A fast, high-temperature process would degrade the char and produce more gas and oil instead.
Setting the Right Temperature for Your Goal
Your target temperature should be dictated entirely by your primary objective. Before starting any pyrolysis operation, you must define what success looks like for your specific feedstock and business model.
- If your primary focus is maximizing liquid fuel (pyrolysis oil): Aim for fast pyrolysis in the medium temperature range, typically between 450°C and 650°C, to vaporize and then condense the valuable hydrocarbons.
- If your primary focus is producing high-quality biochar: Use slower pyrolysis at lower temperatures, generally between 400°C and 500°C, to preserve the carbon structure.
- If your primary focus is generating syngas for on-site energy: Opt for higher temperatures, often above 700°C, to ensure complete thermal cracking of the feedstock into combustible gases.
Ultimately, mastering pyrolysis is mastering the precise control of temperature to achieve a predictable outcome.
Summary Table:
| Target Product | Optimal Temperature Range | Key Characteristic |
|---|---|---|
| Biochar (Solid) | 400°C - 500°C | Slow pyrolysis for carbon structure preservation |
| Liquid Fuel (Oil) | 450°C - 650°C | Fast pyrolysis to maximize liquid hydrocarbon yield |
| Syngas (Gas) | >700°C | High-temperature cracking for combustible gas production |
Ready to optimize your pyrolysis process? The precise temperature control of your reactor is the key to maximizing the value of your output—whether it's biochar, liquid fuel, or syngas. At KINTEK, we specialize in high-performance lab equipment and consumables designed for rigorous thermal processing research and development. Our experts can help you select or customize a pyrolysis system that delivers the exact temperature profiles you need for your specific feedstock and product goals.
Let's discuss your project requirements and build a solution tailored for your success. Contact our thermal processing specialists today to get started!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What is the importance of melting process? Master the Foundation of Metal Production
- What controls melting point? The Hierarchy of Forces from Ionic Bonds to Intermolecular Attractions
- Can two different materials have the same value of specific heat capacity? Unlocking the Science of Thermal Behavior
- What temperature causes melting? Debinding vs. Melting in Metal Fabrication
- Why does melting require energy? Unlock the Science of Latent Heat and Phase Changes



















