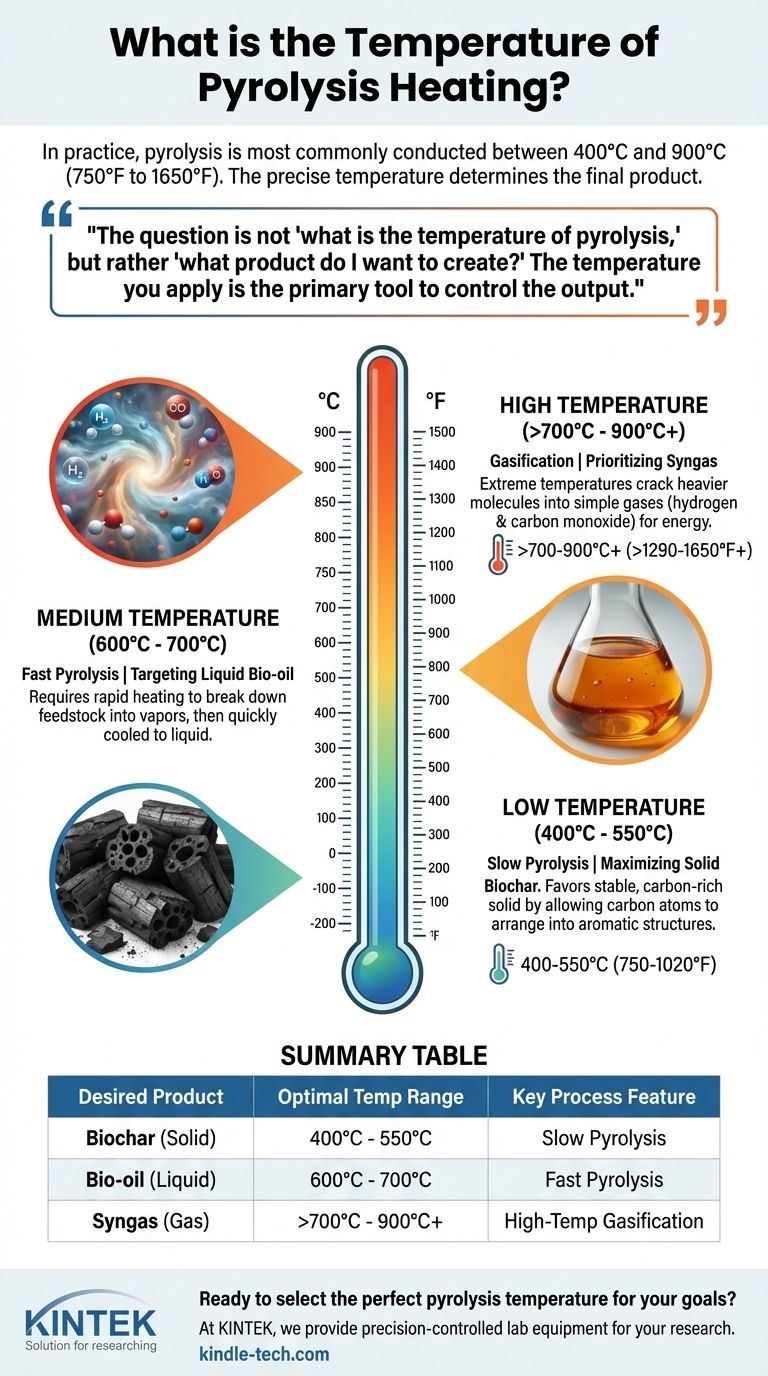
In practice, pyrolysis is most commonly conducted at temperatures between 400°C and 900°C (750°F to 1650°F). This wide range exists because the precise temperature is not a fixed value but a deliberate choice, dialed in to achieve a specific outcome from a specific material. For some organic matter like wood, the process can begin at temperatures as low as 200°C to 300°C.
The question is not "what is the temperature of pyrolysis," but rather "what product do I want to create?" The temperature you apply is the primary tool used to control whether the output is predominantly a solid (biochar), a liquid (bio-oil), or a gas (syngas).

Why Temperature is the Master Variable in Pyrolysis
Pyrolysis is the thermal decomposition of materials in the absence of oxygen. Temperature is the most critical factor influencing the speed of the reaction and the chemical nature of the final products.
The Initial Onset of Pyrolysis
For many materials, the process begins at relatively low temperatures. Wood, for example, starts to pyrolyze between 200–300°C (390–570°F).
At these initial stages, the least stable organic compounds begin to break down, releasing water vapor and other volatile gases.
Low Temperature (Slow Pyrolysis): Maximizing Solid Biochar
When the goal is to produce a stable, carbon-rich solid, a lower temperature range is used, typically between 400°C and 550°C.
This process, often called slow pyrolysis, uses a slower heating rate. It favors the formation of charcoal or biochar by allowing carbon atoms to arrange themselves into stable, aromatic structures rather than breaking apart into smaller gas or liquid molecules.
Medium Temperature (Fast Pyrolysis): Targeting Liquid Bio-oil
To maximize the yield of liquid products, known as bio-oil or tar, a moderate temperature range is employed, generally between 600°C and 700°C.
This process requires very rapid heating to break down the feedstock into vapors. These vapors are then quickly cooled and condensed into a liquid. The speed prevents the larger molecules from further breaking down into gas.
High Temperature (Gasification): Prioritizing Syngas
At high temperatures, typically above 700°C and up to 900°C or more, the primary output becomes non-condensable gases.
These extreme temperatures provide enough energy to crack the heavier liquid and tar molecules into simple, small gas molecules. The resulting product is known as syngas, a mixture of hydrogen and carbon monoxide that can be used for generating energy.
Understanding the Trade-offs of Temperature Control
Choosing a temperature is an engineering decision that involves balancing competing factors. There is no single "best" temperature, only the optimal one for a specific objective.
Energy Input vs. Product Value
Achieving and maintaining higher temperatures requires a significantly greater energy input. This operational cost must be justified by the economic value of the end product. Producing high-value syngas may warrant the high energy cost, whereas producing lower-value biochar would not.
Feedstock and Process Sensitivity
The ideal temperature profile is highly dependent on the feedstock. Plastics, biomass, tires, and municipal solid waste all have different chemical compositions and will yield different product distributions at the same temperature.
Equipment and Complexity
Higher-temperature reactors demand more robust, expensive materials and sophisticated control systems to operate safely and efficiently. The stress on equipment increases exponentially with temperature, impacting maintenance costs and system longevity.
Selecting the Right Temperature for Your Goal
Your choice of temperature should be a direct reflection of your desired outcome. Use these guidelines to determine your ideal operational window.
- If your primary focus is producing solid biochar for soil amendment or filtration: You will operate at lower temperatures, typically in the 400°C to 550°C range with a slow heating process.
- If your primary focus is generating liquid bio-oil as a potential fuel or chemical feedstock: You will use fast pyrolysis methods at moderate temperatures, often between 600°C and 700°C.
- If your primary focus is creating syngas for energy generation or chemical synthesis: You will need high temperatures, generally above 700°C, to maximize gas yield and minimize residual liquids and solids.
Ultimately, controlling the temperature is how you steer the pyrolysis reaction to create the exact product you need.
Summary Table:
| Desired Product | Optimal Temperature Range | Key Process Feature |
|---|---|---|
| Biochar (Solid) | 400°C - 550°C | Slow Pyrolysis |
| Bio-oil (Liquid) | 600°C - 700°C | Fast Pyrolysis |
| Syngas (Gas) | >700°C - 900°C+ | High-Temperature Gasification |
Ready to select the perfect pyrolysis temperature for your specific feedstock and product goals?
At KINTEK, we specialize in providing robust, precision-controlled lab equipment for pyrolysis research and development. Whether you're optimizing for biochar production, bio-oil yield, or syngas generation, our reactors are engineered for reliable performance and precise temperature control.
We serve laboratories and research facilities focused on waste valorization, bioenergy, and sustainable materials. Let our expertise help you achieve your project's objectives efficiently and safely.
Contact KINTEK today to discuss your pyrolysis application and find the ideal equipment solution for your needs.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What is the energy efficiency of biomass? Understanding the 20-40% Range for Power Generation
- What furnace is used for calcination? A Guide to Muffle, Rotary Kiln, and Shaft Furnaces
- What is the process of carbon reactivation? A Sustainable Solution for Spent Activated Carbon
- What is biochar processing? Mastering Slow Pyrolysis for Soil Enhancement & Carbon Sequestration
- What is temperature controlled pyrolysis? Master Heat to Turn Waste into Fuel, Char, or Gas
- What is a furnace for firing pottery called? Discover the Kiln: Your Key to Perfect Ceramics
- What is the principle of rotary kiln? Mastering Continuous Thermal Processing
- What is the regeneration temperature of activated carbon? Optimize Your Process with the Right Method



















