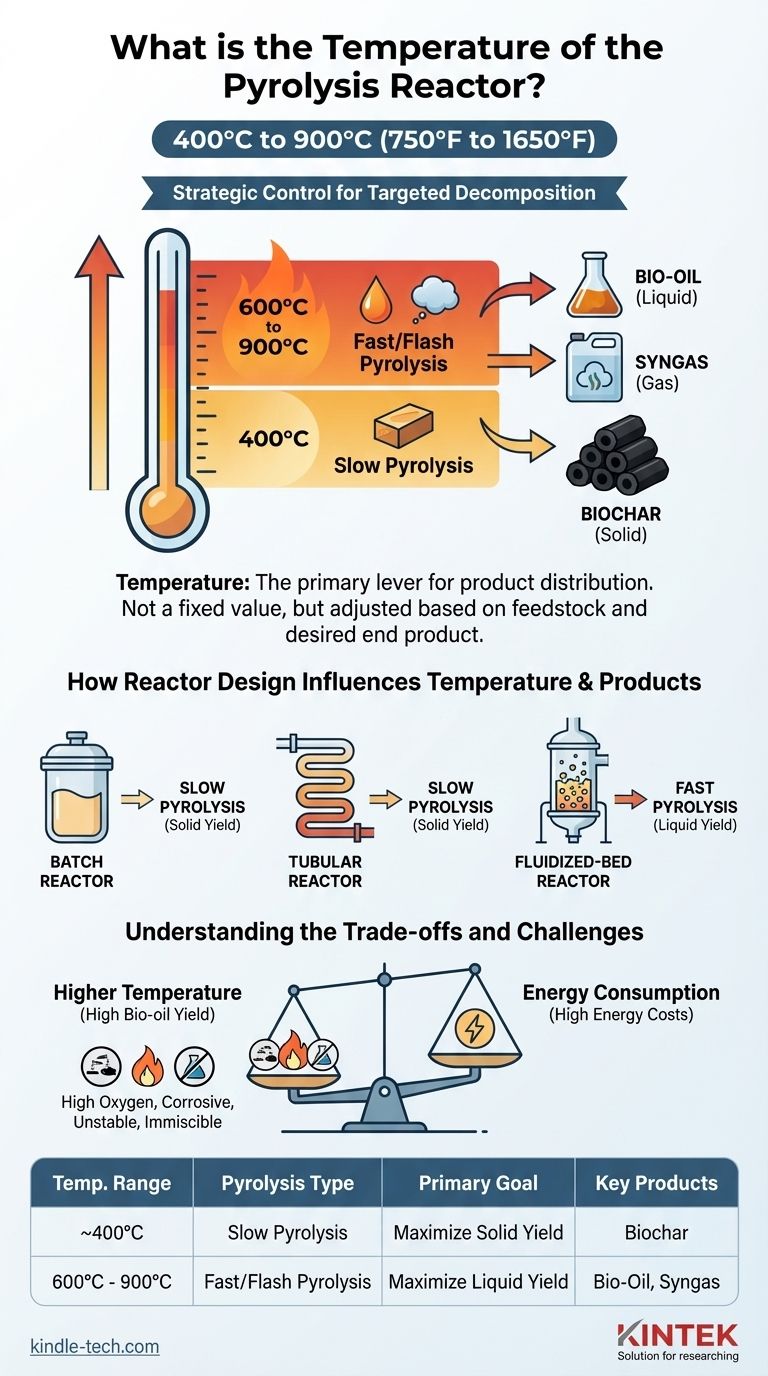
As a general rule, a pyrolysis reactor operates at temperatures between 400°C and 900°C (750°F to 1650°F). The specific temperature is not a fixed value but is the most critical control parameter in the process. It is deliberately adjusted based on the type of raw material (feedstock) and the desired end products, such as bio-oil, biochar, or synthesis gas.
The temperature of a pyrolysis reactor is not a single number but a strategic choice. It is the primary lever used to control the chemical decomposition of a material, directly determining whether the process yields more solid, liquid, or gaseous products.

Why Temperature is the Central Control Variable
Pyrolysis is fundamentally a process of thermal decomposition. It uses high heat in an oxygen-free environment to break down complex materials into simpler, more valuable substances. Temperature, along with the heating rate, dictates exactly how these chemical bonds break.
The Goal: Targeted Decomposition
The heat provides the energy needed to break the chemical bonds within the feedstock. Different types of bonds require different amounts of energy to break.
By controlling the reactor temperature, you control which bonds are broken and how the resulting molecules reform. This gives you direct influence over the final product distribution.
Low vs. High Temperatures
A simple principle governs the outcome: lower temperatures favor the creation of solids, while higher temperatures favor liquids and gases.
Slow pyrolysis, typically at the lower end of the range (around 400°C), is conducted over a longer period. This gentle heating maximizes the production of biochar, a stable, carbon-rich solid.
Fast and flash pyrolysis use much higher temperatures (often 600°C to 900°C) and extremely rapid heating. This process violently shatters the material's molecules to maximize the yield of pyrolysis oil (bio-oil) and synthesis gas.
How Reactor Design Influences Temperature
The type of reactor used is critical because each design is optimized for a different method of heat transfer. The reactor’s design determines how efficiently and quickly it can bring the feedstock to the target temperature.
Batch Reactors for Stability
A batch reactor is a simple, sealed vessel. It is ideal for processes where precise, rapid temperature changes are less important than overall energy stability.
This design is well-suited for slow pyrolysis, where the feedstock can be heated gradually to produce biochar.
Tubular Reactors for Versatility
A tubular reactor allows for a continuous flow of material. While it can be adapted for various pyrolysis types, its design and lower operational costs often make it a good fit for slow pyrolysis.
Fluidized-Bed Reactors for Speed
Reactors like fluidized-bed systems are engineered for extremely efficient heat transfer. They suspend the feedstock particles in a hot fluid (gas), ensuring every particle heats up almost instantly.
This capability is essential for fast pyrolysis, where maximizing the yield of liquid bio-oil is the primary objective.
Understanding the Trade-offs and Challenges
Choosing an operating temperature is a balancing act between desired output, product quality, and operational cost. There is no single "best" temperature, only the right temperature for a specific goal.
The Product Quality Dilemma
While higher temperatures can yield more bio-oil, this liquid fuel is not without its problems. Pyrolysis oil often has a high oxygen content, which makes it corrosive, thermally unstable, and immiscible with conventional fuels.
These characteristics mean it often requires significant upgrading before it can be used as a drop-in replacement for petroleum products.
Energy Consumption
Maintaining temperatures up to 900°C is highly energy-intensive. The cost of this energy is a major factor in the economic viability of a pyrolysis operation.
Higher temperature processes must produce a sufficiently valuable product to justify the increased operational expense.
Making the Right Choice for Your Goal
The optimal temperature is dictated entirely by your primary objective. By understanding the relationship between heat and the final product, you can configure the process to meet your specific needs.
- If your primary focus is maximizing biochar production: Use lower temperatures (around 400°C) and a slower heating rate, characteristic of slow pyrolysis.
- If your primary focus is maximizing bio-oil (liquid fuel) yield: Use higher temperatures (600°C and above) and a very rapid heating rate, which requires a reactor designed for fast pyrolysis.
- If your primary focus is minimizing operational costs: Simpler reactor designs like batch or tubular systems, often used for slow pyrolysis, typically have lower construction and operational expenses.
Ultimately, mastering pyrolysis is about mastering the precise application of heat to transform materials effectively.
Summary Table:
| Temperature Range | Pyrolysis Type | Primary Goal | Key Products |
|---|---|---|---|
| ~400°C | Slow Pyrolysis | Maximize Solid Yield | Biochar |
| 600°C - 900°C | Fast/Flash Pyrolysis | Maximize Liquid Yield | Bio-Oil, Syngas |
Ready to optimize your pyrolysis process? The precise temperature control of your reactor is the single most important factor for achieving your target product yields, whether it's biochar, bio-oil, or syngas. At KINTEK, we specialize in providing the robust lab equipment and expert support you need to master thermal decomposition.
Our team can help you select the right reactor design for your specific feedstock and production goals. Contact us today to discuss how we can enhance your pyrolysis efficiency and product quality.
Get in touch with our experts now!
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Graphite Vacuum Continuous Graphitization Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
People Also Ask
- How does a rotary kiln operate? Master Continuous High-Temperature Processing
- Can activated carbon be burned? Understanding the Risks and Conditions for Combustion
- What is the process of lignocellulosic biomass pretreatment? Unlock the Value in Plant Matter
- What is the difference between biochar gasification and pyrolysis? Unlock the Right Thermal Process for Your Biomass
- What is the composition of wood pyrolysis gas? A Guide to Syngas Production & Control
- What is the technique of pyrolysis? A Guide to Thermal Decomposition Without Oxygen
- What temperature is a carbon regeneration kiln? Master the 650°C-800°C Range for Optimal Results
- What is the refractory material of a rotary kiln? Choose the Right Lining for Efficiency & Durability



















