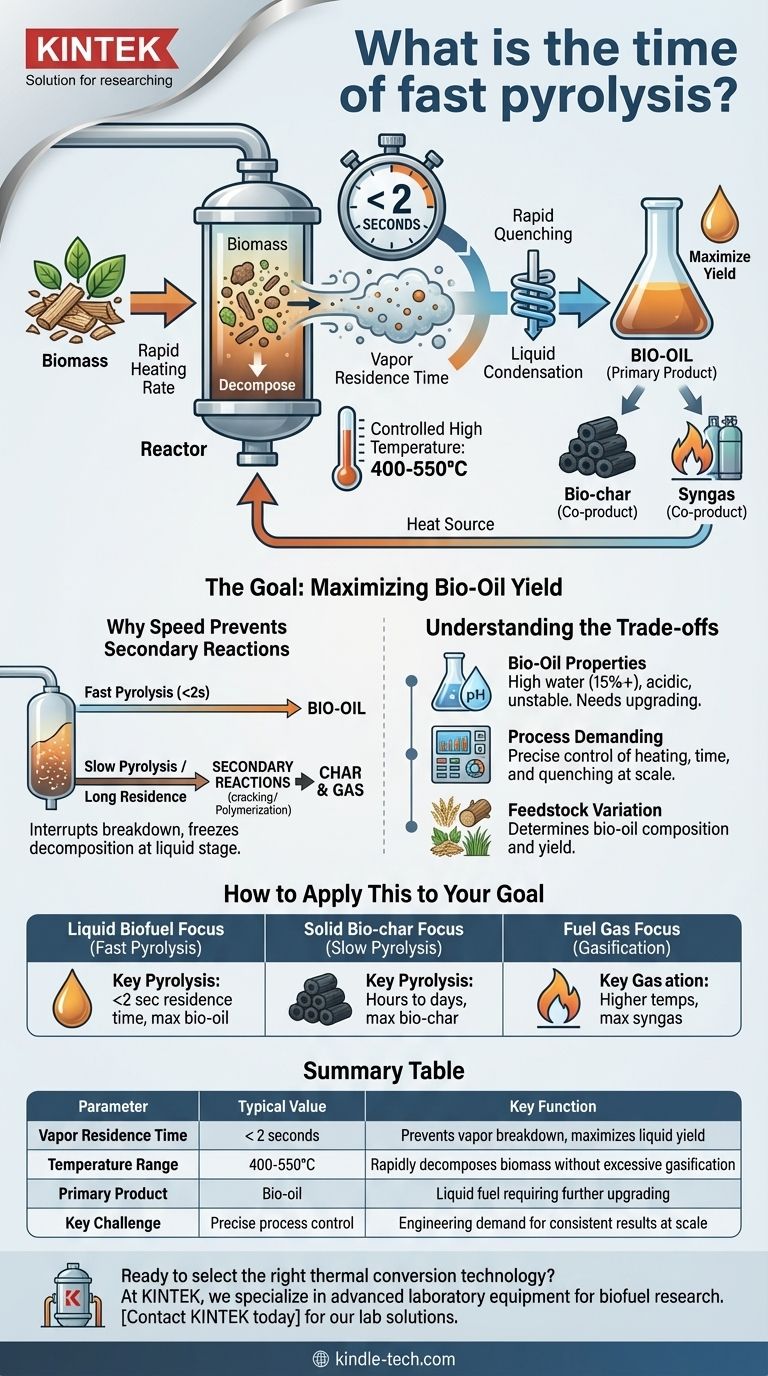
In fast pyrolysis, the residence time for biomass vapors inside the reactor is exceptionally brief, typically lasting less than 2 seconds. This extremely short duration is the defining characteristic of the process, intentionally designed to maximize the yield of liquid bio-oil by rapidly cooling the vapors before they can break down into other products.
The core principle is not just speed for its own sake. The rapid heating and extremely short reaction time are precisely controlled to "freeze" the chemical decomposition at the liquid stage, preventing the valuable vapors from degrading into less desirable gases and solid char.

What Defines the "Fast" in Fast Pyrolysis?
To understand fast pyrolysis, you must look at a set of interconnected conditions that work together. The short time is just one piece of a carefully engineered puzzle designed to produce a specific outcome: liquid fuel.
Extremely Short Vapor Residence Time
The most cited parameter is the vapor residence time, which is under 2 seconds. This is the amount of time the hot gases and vapors, freshly released from the biomass, are allowed to exist at high temperatures within the reactor. Minimizing this time is critical.
High Heating Rate
The solid biomass particles must be heated to the target temperature as quickly as possible. This rapid energy transfer ensures that the entire particle breaks down uniformly, promoting the formation of vapors that will become bio-oil.
Controlled High Temperature
Fast pyrolysis operates within a specific temperature range, typically 400-550°C. This temperature is high enough to rapidly decompose the cellulose, hemicellulose, and lignin in the biomass but is carefully controlled to avoid favoring the creation of gas, which occurs at much higher temperatures.
Rapid Quenching
Immediately after their short residence time in the reactor, the hot vapors must be cooled down (quenched) very quickly. This rapid cooling condenses the vapors into a liquid—the bio-oil—before they can undergo secondary reactions.
The Goal: Maximizing Bio-Oil Yield
The entire process is optimized for one primary purpose: to convert solid biomass into a transportable, storable liquid. The speed of the process is the key to achieving this.
Why Speed Prevents Secondary Reactions
If the hot vapors from the initial biomass decomposition are allowed to remain at high temperatures for too long, they will continue to react. These secondary reactions crack the complex organic molecules into simpler, non-condensable gases (like methane and carbon monoxide) or cause them to re-polymerize into solid char. Fast pyrolysis effectively interrupts this process.
The Resulting Product Mix
While the goal is liquid, fast pyrolysis always produces three products:
- Bio-oil: The primary liquid product, typically representing the highest yield.
- Bio-char: A solid, carbon-rich charcoal co-product.
- Syngas: A mix of non-condensable, flammable gases.
Crucially, the syngas produced can be redirected and burned to provide the heat required for the reactor, making the process partially self-sustaining.
Understanding the Trade-offs
While effective, fast pyrolysis is not a perfect solution. It involves clear engineering challenges and produces a product that requires further processing.
Bio-Oil Is Not Crude Oil
The resulting bio-oil has a high water content (often over 15%) and is acidic and unstable. It cannot be used directly as a "drop-in" fuel in conventional engines and must be upgraded in a process similar to petroleum refining, which adds cost and complexity.
Process Control is Demanding
Achieving the precise conditions—high heating rates, short residence times, and rapid quenching—at a large, commercial scale is a significant engineering challenge. The process is sensitive to variations in temperature, pressure, and feedstock.
Feedstock Determines Output
The exact chemical composition and yield of the bio-oil can vary significantly depending on the type of biomass used (e.g., wood, agricultural waste, grasses) and the specific operating conditions of the reactor.
How to Apply This to Your Goal
Choosing a thermal conversion technology depends entirely on your desired end product.
- If your primary focus is liquid biofuel: Fast pyrolysis is the optimal pathway because its short residence time is specifically designed to maximize bio-oil yield.
- If your primary focus is solid bio-char: You would choose slow pyrolysis, which uses much longer residence times (hours to days) to intentionally promote the formation of a stable, carbon-rich solid.
- If your primary focus is producing fuel gas (syngas): You would use gasification, which involves even higher temperatures and specific conditions to convert nearly all the biomass into a gaseous product.
By understanding the critical role of time, you can select the right process to convert biomass into the specific valuable product you need.
Summary Table:
| Parameter | Typical Value | Key Function |
|---|---|---|
| Vapor Residence Time | < 2 seconds | Prevents vapor breakdown, maximizes liquid yield |
| Temperature Range | 400-550°C | Rapidly decomposes biomass without excessive gasification |
| Primary Product | Bio-oil | Liquid fuel requiring further upgrading |
| Key Challenge | Precise process control | Engineering demand for consistent results at scale |
Ready to select the right thermal conversion technology for your biomass project?
At KINTEK, we specialize in advanced laboratory equipment for biofuel research and development. Whether you are optimizing fast pyrolysis conditions for maximum bio-oil yield or exploring other pathways like slow pyrolysis or gasification, our precise heating systems and reactors are designed for reliability and control.
Let our experts help you achieve your specific product goals. Contact KINTEK today to discuss how our lab solutions can accelerate your bioenergy innovations.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products



















