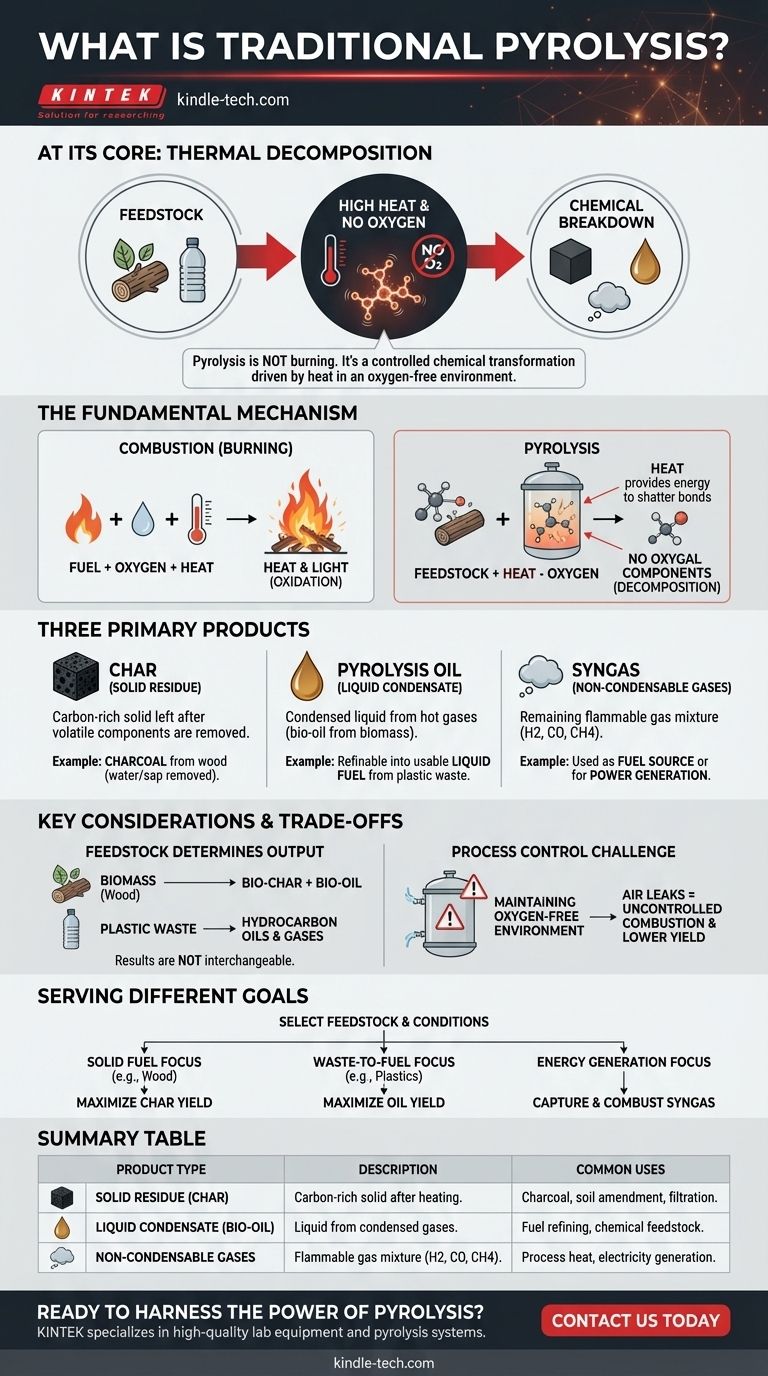
At its core, traditional pyrolysis is a process of thermal decomposition. It uses high temperatures to break down materials like wood or plastic in an environment with little to no oxygen. This key difference—the absence of oxygen—prevents the material from burning and instead forces it to break apart into new, simpler chemical substances.
Pyrolysis isn't burning; it's a controlled chemical transformation driven by heat. By removing oxygen, we prevent combustion and instead force complex organic molecules to decompose into a mixture of useful gases, liquids, and a solid carbon-rich residue.

The Fundamental Mechanism: Heat vs. Combustion
What "Thermal Decomposition" Means
Pyrolysis uses intense heat to provide the energy needed to shatter the strong chemical bonds holding large, complex molecules together.
The input material, known as feedstock, doesn't react with oxygen as it would in a fire. Instead, the molecules simply vibrate until they break into smaller, more stable fragments.
The Critical Role of an Oxygen-Free Environment
Combustion (burning) is an oxidation reaction that rapidly releases energy. It requires fuel, oxygen, and heat.
Pyrolysis removes the oxygen from this equation. Without it, the feedstock cannot ignite. This forces a different chemical pathway, allowing us to capture the resulting chemical components instead of just releasing their energy as heat and light.
The Three Primary Products of Pyrolysis
The specific output depends heavily on the feedstock and process conditions, but pyrolysis generally creates three distinct product types.
The Solid Residue (Char)
This is the carbon-rich solid material left after the volatile components have been driven off by the heat.
A classic example is the production of charcoal from wood. The pyrolysis process removes water, sap, and other compounds, leaving a porous, high-carbon solid.
The Liquid Condensate (Pyrolysis Oil)
When the hot gases produced during pyrolysis are cooled, a portion of them condenses into a liquid. This is often called bio-oil (from biomass) or pyrolysis oil.
This liquid is a complex mixture of different organic compounds. As noted in the example of plastic waste, this oil can often be refined into a usable liquid fuel.
The Non-Condensable Gases (Syngas)
The remaining gases that do not turn into liquid upon cooling are often referred to as syngas (synthesis gas).
This gas is a mixture of components like hydrogen, carbon monoxide, carbon dioxide, and methane. It is flammable and can be used as a fuel source, sometimes to help power the pyrolysis process itself.
Key Considerations and Trade-offs
Feedstock Determines the Output
The single most important variable is the material you start with.
Pyrolyzing biomass like wood will produce bio-char and bio-oil. Pyrolyzing plastic waste, which is derived from petroleum, will produce a very different set of hydrocarbon oils and gases. The results are not interchangeable.
The Challenge of Process Control
The primary technical challenge in pyrolysis is maintaining the oxygen-free environment at high temperatures.
Any air leaks into the reactor can cause partial, uncontrolled combustion. This lowers the yield of desired products and can create hazardous operating conditions.
How Pyrolysis Serves Different Goals
The specific outputs of pyrolysis can be targeted by carefully selecting the feedstock and controlling the process conditions.
- If your primary focus is solid fuel or carbon sequestration: You would use a biomass feedstock like wood and optimize conditions to maximize the yield of stable, solid charcoal.
- If your primary focus is waste-to-fuel conversion: You would use a feedstock like waste plastics or tires to produce a high yield of liquid pyrolysis oil that can be refined.
- If your primary focus is energy generation: You could design a system to capture and combust the syngas produced, using it to generate electricity or provide process heat.
Ultimately, pyrolysis offers a powerful thermal method for deconstructing materials to capture their underlying chemical value.
Summary Table:
| Product Type | Description | Common Uses |
|---|---|---|
| Solid Residue (Char) | Carbon-rich solid left after heating. | Charcoal, soil amendment, filtration. |
| Liquid Condensate (Bio-Oil) | Liquid from condensed pyrolysis gases. | Fuel refining, chemical feedstock. |
| Non-Condensable Gases (Syngas) | Flammable gas mixture (H2, CO, CH4). | Process heat, electricity generation. |
Ready to harness the power of pyrolysis in your lab? KINTEK specializes in high-quality lab equipment, including pyrolysis reactors and related systems, to help you efficiently convert biomass or waste materials into valuable products. Our experts can help you select the right equipment to meet your research or production goals. Contact us today to discuss your specific application and see how we can support your work!
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- Why are high temperatures required when sintering stainless steels? Unlock Pure, High-Density Results
- How is a high-temperature calcination furnace utilized in BZY20 Sol-gel? Achieve Pure Cubic Perovskite Phases
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies



















