In principle, nearly any material can be evaporated, but the feasibility depends on the conditions required. In industrial and scientific applications, this process is most commonly applied to a vast range of metals, ceramics, and dielectric compounds, particularly those with high melting temperatures that can be vaporized efficiently in a vacuum.
The critical factor isn't whether a material can evaporate, but whether it can be turned into a vapor at a practical rate and temperature without decomposing. This is why the process is almost always performed in a vacuum, which dramatically lowers the required temperature.

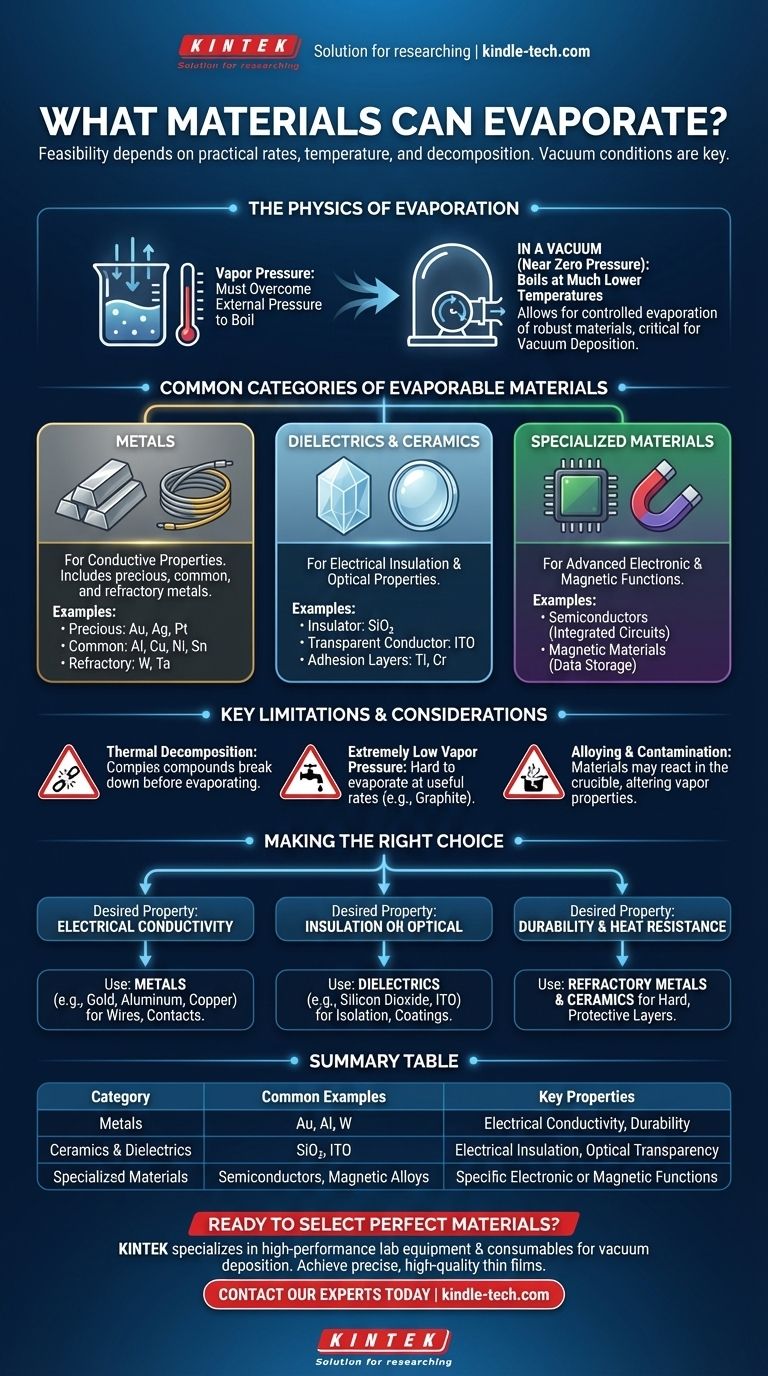
The Physics of Evaporation
Vapor Pressure is Key
Evaporation is the transition of a substance from a solid or liquid state into a gas. For this to happen, a material's atoms or molecules must gain enough energy to overcome the forces holding them together.
The tendency of a material to evaporate at a given temperature is called its vapor pressure. When a material's vapor pressure equals the surrounding pressure, it boils.
The Role of Vacuum
In a vacuum, the surrounding pressure is near zero. This allows materials to "boil" or evaporate at much lower temperatures than they would at normal atmospheric pressure.
This is the central principle behind vacuum deposition, a technology used to create ultra-thin coatings for electronics, optics, and tools. It allows for the controlled evaporation of even very robust materials.
Common Categories of Evaporable Materials
The references focus on materials used in thin-film deposition, a primary application of controlled evaporation. These materials are chosen for specific electrical, optical, or physical properties.
Metals
Metals are widely used for their conductive properties. The process can handle everything from common metals to those with extremely high melting points.
- Precious Metals: Gold (Au), Silver (Ag), and Platinum (Pt) are excellent conductors that resist corrosion.
- Common Metals: Aluminum (Al), Copper (Cu), Nickel (Ni), and Tin (Sn) are used for general-purpose conductive layers and contacts.
- Refractory Metals: Tungsten (W) and Tantalum (Ta) have very high melting points and are used for applications requiring durability and heat resistance.
Dielectrics and Ceramics
These materials are typically electrical insulators or have specific optical properties. They are essential for building complex electronic and optical components.
- Silicon Dioxide (SiO₂): A fundamental insulator in semiconductor manufacturing.
- Indium Tin Oxide (ITO): A transparent material that also conducts electricity, making it vital for touch screens and solar cells.
- Titanium (Ti) and Chromium (Cr): Often used as adhesion layers to help subsequent material layers stick to a substrate.
Other Material Classes
The versatility of vacuum evaporation extends to other specialized materials critical for modern technology.
- Semiconductors: Materials used to fabricate integrated circuits and microchips.
- Magnetic Materials: Used for data storage and sensor applications.
Key Limitations and Considerations
While the range of materials is vast, not everything is a good candidate for evaporation. The primary challenges are thermal stability and achieving sufficient vapor pressure.
Thermal Decomposition
The most significant limitation is decomposition. Many complex compounds, especially organic ones, will break down or burn when heated before they can build enough vapor pressure to evaporate.
Extremely Low Vapor Pressure
Some materials, like graphite (carbon), have exceptionally strong atomic bonds. Reaching a temperature high enough to evaporate them at a useful rate is technically difficult and energy-intensive.
Alloying and Contamination
When co-evaporating multiple materials, they can sometimes form alloys in the crucible. This can alter the properties of the vapor and the resulting film, requiring careful process control.
Making the Right Choice for Your Application
Selecting a material is driven entirely by the desired properties of the final coating.
- If your primary focus is electrical conductivity: Use metals like gold, silver, copper, or aluminum for creating wires, contacts, or reflective surfaces.
- If your primary focus is insulation or optical properties: Use dielectric materials like silicon dioxide for electrical isolation or indium tin oxide for transparent conductive coatings.
- If your primary focus is durability and heat resistance: Use refractory metals like tungsten, tantalum, or ceramics to create hard, protective layers.
Ultimately, material selection for evaporation is a balance between the desired final properties and the physical constraints of the process itself.
Summary Table:
| Material Category | Common Examples | Key Properties |
|---|---|---|
| Metals | Gold (Au), Aluminum (Al), Tungsten (W) | Electrical Conductivity, Durability |
| Ceramics & Dielectrics | Silicon Dioxide (SiO₂), Indium Tin Oxide (ITO) | Electrical Insulation, Optical Transparency |
| Specialized Materials | Semiconductors, Magnetic Alloys | Specific Electronic or Magnetic Functions |
Ready to select the perfect evaporable materials for your project?
KINTEK specializes in high-performance lab equipment and consumables for vacuum deposition. Whether you're working with conductive metals, optical dielectrics, or specialized alloys, our expertise ensures you achieve precise, high-quality thin films.
Contact our experts today to discuss your specific material and coating requirements and discover how KINTEK can enhance your laboratory's capabilities.
Visual Guide
Related Products
- Lab Blown Film Extrusion Three Layer Co-Extrusion Film Blowing Machine
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Boron Nitride (BN) Ceramic Tube
- Vacuum Cold Trap Chiller Indirect Cold Trap Chiller
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- What is step coverage in thermal evaporation? Avoid Device Failure with the Right Deposition Method
- What materials are used in vacuum evaporation? A Guide to Metals, Alloys, and Dielectrics
- What is thermal evaporation technique? A Guide to Simple, Cost-Effective Thin-Film Deposition
- What are the applications of evaporation in industries? From Wastewater to Electronics
- What is vacuum deposition process? Achieve High-Performance Coatings with Precision
- What is thermal evaporation deposition for thin films? A Guide to Simple, Cost-Effective Coating
- Can you evaporate silver? Master the PVD Process for High-Performance Coatings
- What is the source of evaporation for thin film? Choosing Between Thermal and E-Beam Methods



















