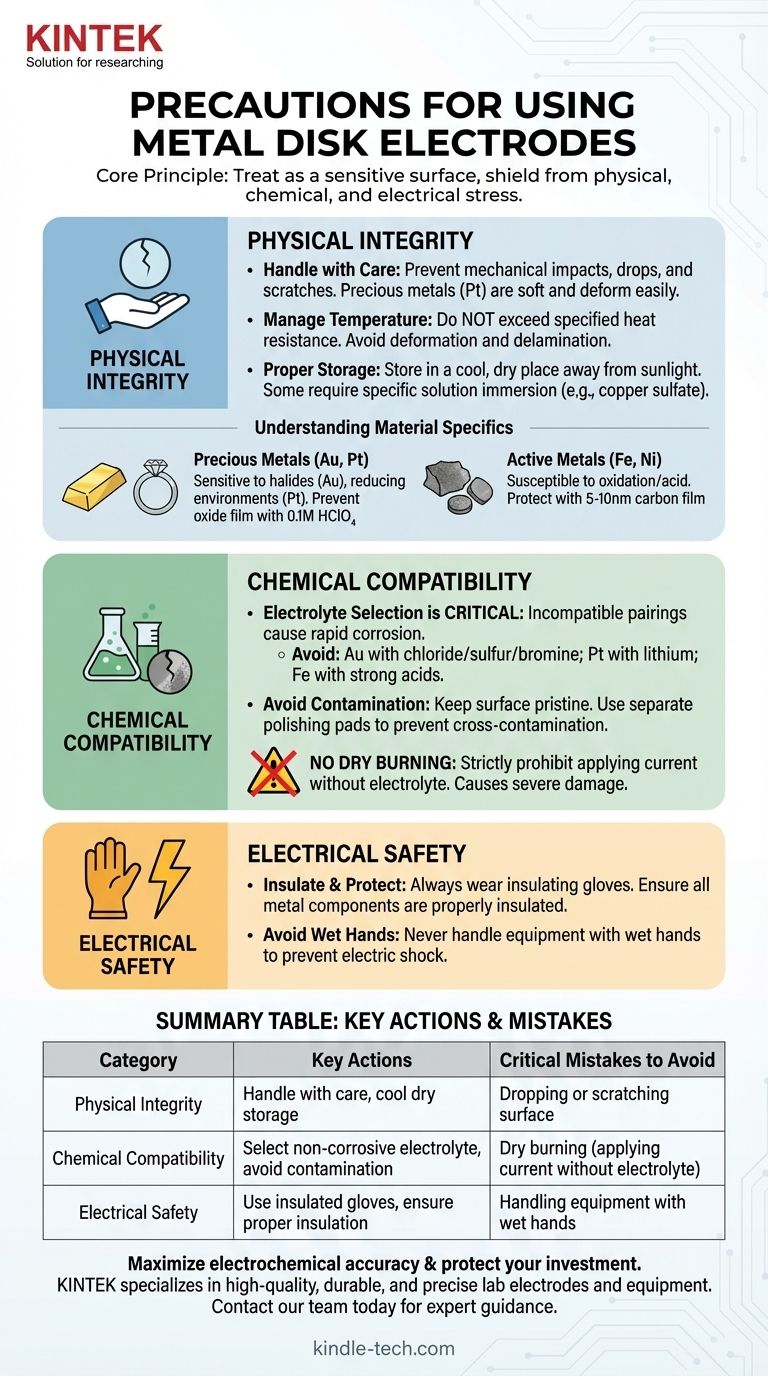
To properly use metal disk electrodes, you must implement precautions across three domains: physical integrity, chemical compatibility, and electrical safety. Key actions include careful handling to prevent mechanical damage, selecting an electrolyte that will not corrode the specific electrode material, never applying current without an electrolyte ("dry burning"), and using appropriate insulation and gloves to prevent electric shock.
Metal disk electrodes are precision instruments whose accuracy and lifespan depend entirely on preventative care. The core principle is to treat the electrode not as a durable tool, but as a sensitive surface that must be shielded from physical, chemical, and electrical stress at all times.

Protecting Physical Integrity
The performance of an electrode is directly tied to the condition of its surface and structure. Physical damage is often irreversible.
Handling and Mechanical Shock
The electrode surface is fragile. Mechanical impacts, such as collisions, drops, or scratches from hard objects, can permanently alter its electrochemical properties.
Precious metal electrodes, like platinum, are particularly soft and can be easily scratched or deformed, affecting surface area calculations and experimental reproducibility.
Managing Temperature
High-temperature experiments must not exceed the electrode's specified heat resistance. Excessive heat can cause materials to deform or delaminate, leading to catastrophic failure.
Proper Storage
When not in use, electrodes should be stored in a cool, dry place away from direct sunlight. Some may require immersion in a specific solution, such as saturated copper sulfate, to maintain surface stability.
Preventing Chemical Degradation
Chemical reactions are the purpose of an electrode, but unintended reactions can quickly destroy it.
The Critical Role of Electrolyte Selection
Choosing the correct electrolyte is the single most important chemical precaution. Incompatible pairings lead to rapid corrosion and invalidate results.
Well-known incompatibilities include:
- Gold (Au) with electrolytes containing chloride, sulfur, or bromine.
- Platinum (Pt) with solutions containing lithium.
- Iron (Fe) with strong acids.
Avoiding Surface Contamination
The electrode surface must be kept pristine. Prevent contact with organic substances that can foul the surface.
When polishing, never use the same polishing pad for different grades of polishing powder. This practice causes cross-contamination that embeds particles into the electrode surface.
The Dangers of Dry Burning
Strictly prohibit dry burning—the application of current to the electrode without it being immersed in an electrolyte. This can cause immediate and severe damage to the electrode surface.
Understanding the Trade-offs and Material Specifics
Different materials require different precautions, and maintenance procedures come with their own risks.
Precious Metals (Gold, Platinum)
While relatively inert, these materials are expensive and have specific vulnerabilities. Gold is sensitive to halides, while platinum can be affected in strongly reducing environments.
To prevent the formation of an oxide film on precious metal electrodes, it is often recommended to immerse them in a solution like 0.1M HClO₄.
Active Metals (Iron, Nickel)
These metals are more susceptible to oxidation and acid attack. An effective protective measure is applying a thin, 5-10nm vacuum-coated carbon film to the surface.
Maintenance and Calibration
Regular maintenance is non-negotiable. Visually inspect the electrode for damage and periodically check its electrical resistance.
Calibrate the electrode regularly by measuring its output in a standard solution of known potential. Significant deviation signals a problem with the electrode's surface or integrity.
Making the Right Choice for Your Goal
Adhering to these precautions will protect your investment and ensure the validity of your data. Tailor your focus based on your immediate priority.
- If your primary focus is experimental accuracy: Prioritize meticulous surface polishing with separate pads and perform regular calibration in a standard solution.
- If your primary focus is electrode longevity: Be absolutely certain of your electrolyte-electrode compatibility and always follow proper storage protocols.
- If your primary focus is personal safety: Always wear insulating gloves, ensure all metal components are properly insulated, and never handle equipment with wet hands.
Consistent and careful handling is the foundation of reliable and reproducible electrochemical research.
Summary Table:
| Precaution Category | Key Actions | Critical Mistakes to Avoid |
|---|---|---|
| Physical Integrity | Handle with care; store in a cool, dry place. | Dropping or scratching the electrode surface. |
| Chemical Compatibility | Select a non-corrosive electrolyte; avoid surface contamination. | Dry burning (applying current without electrolyte). |
| Electrical Safety | Use insulated gloves; ensure proper insulation. | Handling equipment with wet hands. |
Maximize your lab's electrochemical accuracy and protect your electrode investment. KINTEK specializes in high-quality lab equipment and consumables, including electrodes designed for durability and precision. Our experts can help you select the right equipment and provide guidance on best practices. Contact our team today to ensure your experiments are safe, reliable, and reproducible!
Visual Guide
Related Products
- Platinum Auxiliary Electrode for Laboratory Use
- Ultra-Vacuum Electrode Feedthrough Connector Flange Power Electrode Lead for High-Precision Applications
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
People Also Ask
- Why is a platinum wire (PtW) counter electrode preferred for cathode LSV tests? Ensure High-Precision Research
- Why is platinum a good counter electrode? For Superior Chemical Inertness and Electron Transfer
- What are the advantages of using a Platinum (Pt) electrode for zirconium testing? Ensure High-Precision Data Integrity
- Why is platinum typically selected as the auxiliary electrode for electrochemical testing of oxazoline inhibitors?
- What is the function of a platinum electrode as an auxiliary electrode? Ensure Precise Nickel Coating Corrosion Testing













