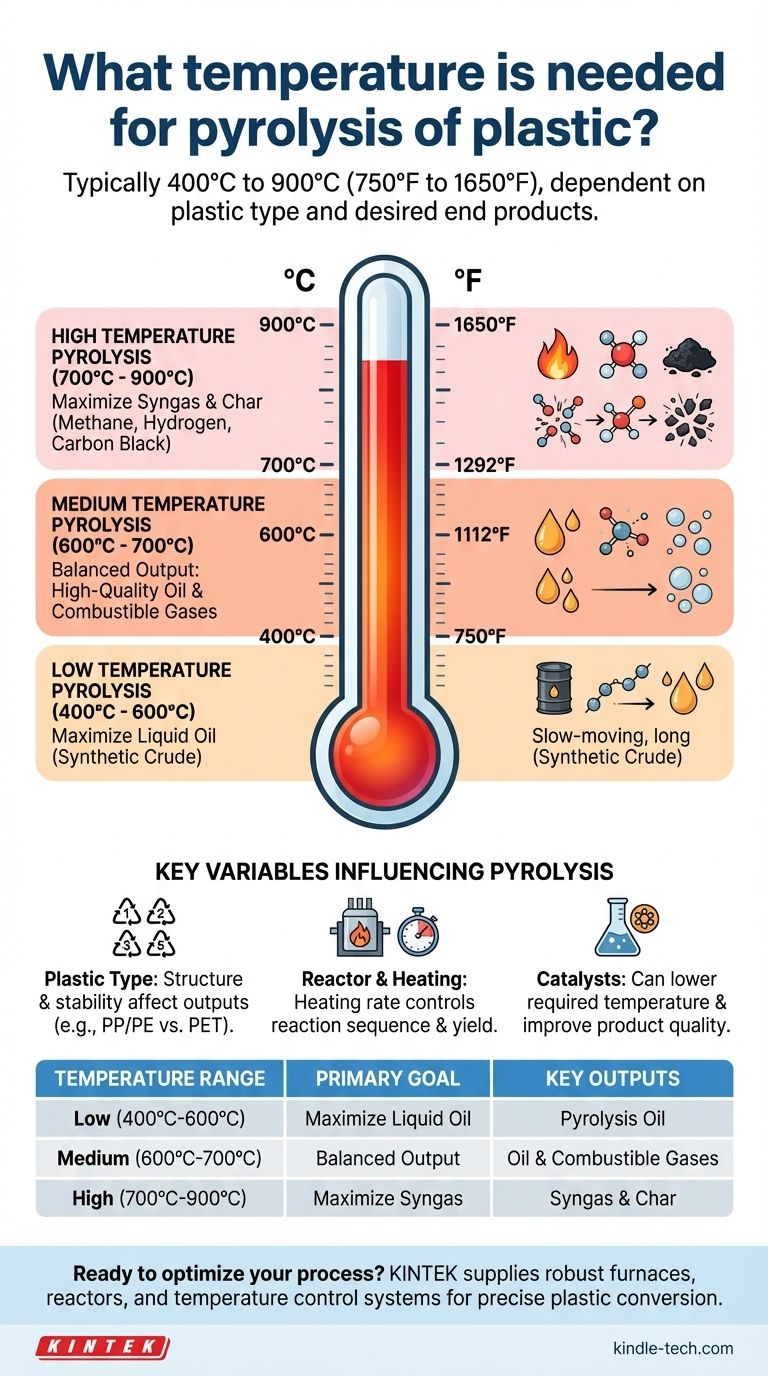
In short, the temperature required for the pyrolysis of plastic typically ranges from 400°C to 900°C (750°F to 1650°F). The exact temperature depends entirely on the type of plastic being processed and the desired end products, such as oil, gas, or char.
Understanding pyrolysis isn't about finding a single magic number for temperature. Instead, it's about controlling temperature to selectively break down plastic into a specific mix of valuable outputs.

The Role of Temperature in Plastic Pyrolysis
Pyrolysis is the thermal decomposition of a material in the absence of oxygen. When you apply heat to plastics without oxygen, their long polymer chains break apart into smaller, more valuable molecules. Temperature is the primary lever you pull to control what those molecules are.
Low Temperature Pyrolysis (400°C - 600°C)
At the lower end of the spectrum, the process is slower and less intense. The longer polymer chains are broken down, but not as aggressively.
This range is primarily used to maximize the liquid oil yield. Slower decomposition favors the formation of heavier hydrocarbon chains that condense into a synthetic crude oil, often called pyrolysis oil.
Medium Temperature Pyrolysis (600°C - 700°C)
As the temperature increases, the cracking of polymer chains becomes more severe. This is a versatile range often targeted for a balanced output.
You will typically get a mix of high-quality liquid oil and combustible gases. The higher heat breaks down some of the heavier oil fractions into lighter, non-condensable gases like methane, hydrogen, and ethylene.
High Temperature Pyrolysis (700°C - 900°C)
At these very high temperatures, the molecular bonds are shattered aggressively. The primary goal here is often not liquid fuel.
This range is used to maximize the production of combustible gases (syngas) and solid carbon residue (char or carbon black). The intense heat cracks nearly all the hydrocarbon chains into the simplest, gaseous forms.
Understanding the Key Variables
Temperature is the most critical factor, but it doesn't work in isolation. The final output is a result of several interconnected variables.
The Impact of Plastic Type
Different plastics have different chemical structures and thermal stabilities. For example, Polypropylene (PP) and Polyethylene (PE) tend to break down into useful oil and wax fractions at lower temperatures, while PET requires different conditions and can produce more char and gas.
Reactor Design and Heating Rate
How quickly you reach the target temperature (the heating rate) also matters. A slow heating rate allows for more controlled, sequential reactions, often favoring oil production. A rapid heating rate (fast pyrolysis) can produce different yields and is often used to maximize specific chemical outputs.
The Role of Catalysts
Catalysts can be introduced into the process to lower the required temperature or to steer the chemical reactions toward producing a more specific, higher-quality product. For example, a catalyst might help produce a liquid fuel with properties closer to commercial gasoline or diesel.
Making the Right Choice for Your Goal
The optimal temperature is defined by your objective. Before starting any process, you must define what you want to produce.
- If your primary focus is maximizing liquid oil production: Operate in the lower temperature range of 400°C to 600°C, which favors the formation of longer hydrocarbon chains.
- If your primary focus is producing combustible syngas: Operate at the higher temperature range of 700°C to 900°C to aggressively crack the plastics into simple gas molecules.
- If your primary focus is a balanced output of oil and gas: A medium temperature range of 600°C to 700°C provides a versatile middle ground for producing both valuable streams.
Ultimately, controlling the temperature is the key to unlocking the specific value hidden within waste plastics.
Summary Table:
| Temperature Range | Primary Goal | Key Outputs |
|---|---|---|
| Low (400°C - 600°C) | Maximize Liquid Oil | Pyrolysis Oil (Synthetic Crude) |
| Medium (600°C - 700°C) | Balanced Output | High-Quality Oil & Combustible Gases |
| High (700°C - 900°C) | Maximize Syngas | Combustible Gases (e.g., Methane, Hydrogen) & Char |
Ready to build or optimize your plastic pyrolysis process?
Precise temperature control is critical for achieving your target yields of oil, gas, or char. The right lab equipment ensures accurate testing, repeatable results, and scalable processes.
KINTEK specializes in supplying the robust furnaces, reactors, and temperature control systems you need to succeed. Whether you're in R&D or scaling up production, we provide the reliable tools for efficient plastic waste conversion.
Contact our experts today to discuss your specific requirements and find the perfect heating solution for your pyrolysis project.
Visual Guide
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vertical Laboratory Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the use of electric muffle furnace? Achieve Pure, High-Temperature Processing
- What is the thermal debinding process? A Guide to Safe Binder Removal for MIM & Ceramics
- What is the annealing temperature of quartz? Achieve Optimal Thermal Stability for Your Components
- What is the use of furnace in laboratory? Unlock Material Transformation for Your Research
- What is the use of high temperature muffle furnace? Achieve Pure, Contamination-Free Thermal Processing



















