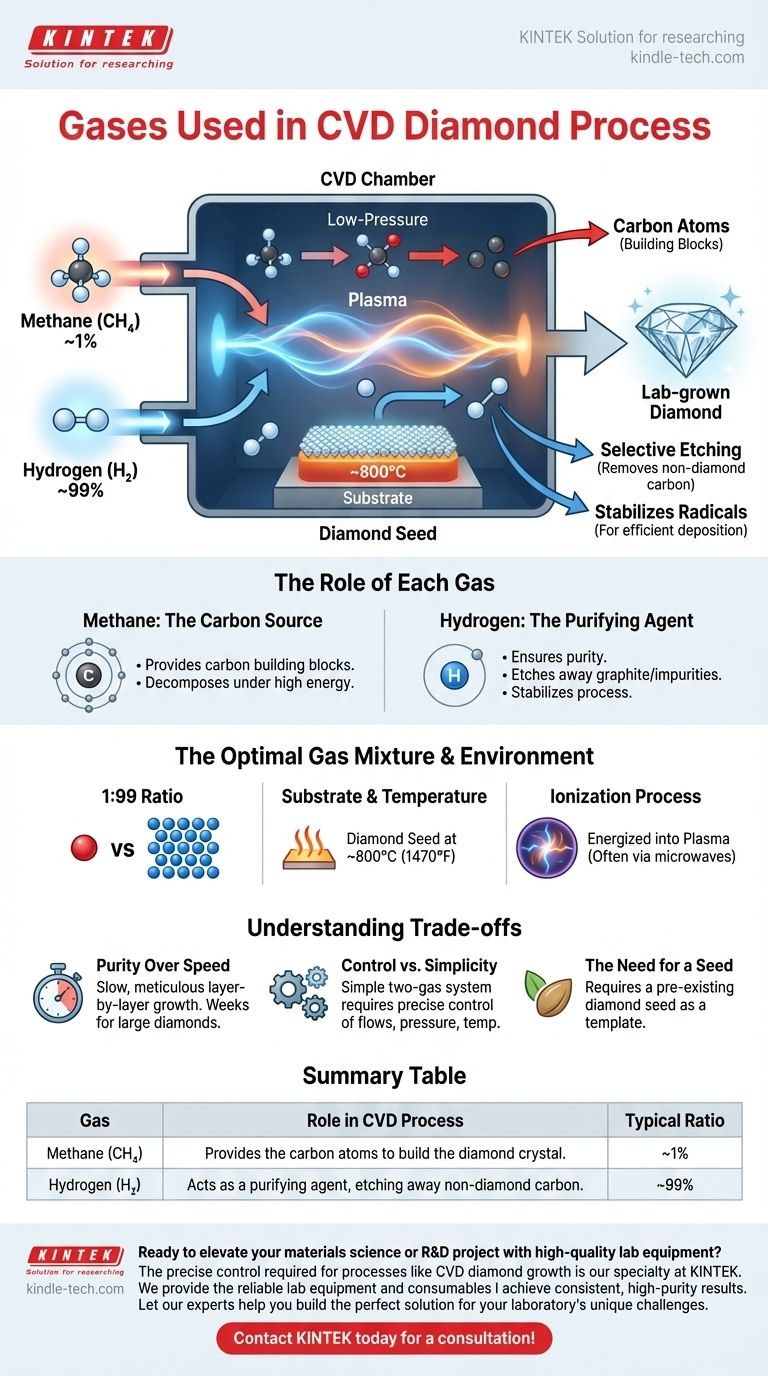
At its core, the chemical vapor deposition (CVD) diamond process relies on a precise and deceptively simple mixture of two primary gases. The most common combination is a carbon-bearing gas, almost always high-purity methane (CH₄), and an overwhelming amount of ultra-high-purity hydrogen (H₂). This mixture is typically maintained in a ratio of approximately 1 part methane to 99 parts hydrogen.
The entire process hinges on a synergistic relationship: methane provides the carbon atoms that build the diamond, while hydrogen acts as a critical quality-control agent, ensuring that only diamond crystals are formed by selectively removing any other form of carbon.

The Role of Each Gas in Diamond Creation
To understand the CVD process, you must see the gases not as a simple mixture, but as two agents with distinct and equally vital jobs. The success of creating a flawless lab-grown diamond depends entirely on how well each performs its role.
The Carbon Source: The Role of Methane
Methane (CH₄) is selected as the primary source of carbon, the fundamental building block of a diamond.
The process begins by introducing this carbon-rich gas into a sealed, low-pressure chamber. When high energy is applied—often via microwaves or a hot filament—the methane molecules break apart, releasing their carbon atoms.
The Purifying Agent: The Role of Hydrogen
Hydrogen (H₂) is the unsung hero of the process. While methane provides the raw material, hydrogen ensures the integrity and purity of the final crystal. Its role is twofold.
First, hydrogen is essential for selective etching. As carbon atoms deposit onto the diamond seed, some may try to form weaker, non-diamond bonds, such as graphite. Hydrogen is far more effective at reacting with and etching away this undesirable carbon, leaving only the strong, diamond-bonded carbon behind.
Second, the hydrogen-rich environment helps create and stabilize the chemically active radicals needed for the deposition to occur efficiently on the heated substrate surface.
The Optimal Gas Mixture
The standard 1:99 ratio of methane to hydrogen is critical. The massive excess of hydrogen is necessary to guarantee that its purifying and etching effect dominates the process.
This overwhelming hydrogen presence ensures that any non-diamond carbon is removed almost as soon as it forms, preventing defects and resulting in a high-purity diamond crystal.
The Environment That Makes It Work
The gases alone do not create a diamond. They must be managed within a highly controlled environment where other factors enable the chemical reactions.
The Substrate and Temperature
The process requires a substrate, typically a small, thin slice of a previously grown diamond, often called a diamond seed. This seed provides the crystalline template for the new carbon atoms to bond to.
This seed is placed in the chamber and heated to a precise temperature, generally around 800°C (1470°F). This heat gives the carbon atoms the energy they need to settle into the rigid diamond lattice.
The Ionization Process
Merely flooding the hot chamber with gas is not enough. The mixture must be energized or ionized into a plasma—a cloud of chemically active particles.
This is the step that breaks down the stable methane and hydrogen molecules, creating the free carbon atoms and reactive hydrogen radicals that drive the layer-by-layer growth of the diamond.
Understanding the Trade-offs
The choice of gases and process parameters involves inherent compromises that define the quality and efficiency of CVD diamond growth.
Purity Over Speed
The heavy use of hydrogen for selective etching makes the process meticulous but slow. Growing a sizable diamond can take several weeks. The priority is forming a perfect crystal lattice, which requires a deliberate, layer-by-layer method rather than rapid, uncontrolled deposition.
Control vs. Simplicity
Using a simple two-gas system of methane and hydrogen allows for extremely fine control over the diamond's final purity and characteristics. However, this demands sophisticated equipment to precisely manage gas flows, low pressures, and stable high temperatures.
The Need for a Seed
This process is one of accretion, not spontaneous creation. A diamond cannot be formed from gases without a pre-existing diamond seed to provide the structural template. The quality of the final product is directly influenced by the quality of the initial seed.
Making the Right Choice for Your Goal
Your understanding of the CVD gas mixture depends on your ultimate objective.
- If your primary focus is achieving maximum purity: The 1:99 methane-to-hydrogen ratio is the most critical variable, as excess hydrogen is the key to etching away defects.
- If your primary focus is understanding the core principle: Remember that you need a carbon source (methane) to provide the building blocks and a purifying agent (hydrogen) to ensure the blocks assemble correctly into a diamond structure.
- If your primary focus is process efficiency: Realize that controlling the chamber temperature (around 800°C) and the energy source is just as critical as managing the gas composition.
Ultimately, the creation of a gem-quality diamond in a lab is a masterful exercise in controlled chemistry, where simple gases are transformed under precise conditions.
Summary Table:
| Gas | Role in CVD Process | Typical Ratio |
|---|---|---|
| Methane (CH₄) | Provides the carbon atoms to build the diamond crystal. | ~1% |
| Hydrogen (H₂) | Acts as a purifying agent, etching away non-diamond carbon. | ~99% |
Ready to elevate your materials science or R&D project with high-quality lab equipment? The precise control required for processes like CVD diamond growth is our specialty at KINTEK. We provide the reliable lab equipment and consumables you need to achieve consistent, high-purity results. Let our experts help you build the perfect solution for your laboratory's unique challenges.
Contact KINTEK today for a consultation!
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How do the growth patterns of HPHT, CVD, and natural diamonds differ? Uncover the Morphology of Lab vs. Mined Gems
- What is the DC sputtering technique? A Guide to Efficient Metal Thin Film Deposition
- Why is it preferred to use carbon nanotubes as catalysts in chemical industries? Maximize Catalytic Performance & Efficiency
- What are some of the different methods of chemical vapour deposition? Choose the Best CVD Process for Your Application
- What is the primary function of a High Vacuum CVD Furnace? Master High-Quality Graphene Synthesis
- What temperature is maintained in CVD? Unlocking the High-Heat Process for Superior Coatings
- What is the floating catalyst method? A Guide to High-Yield CNT Production
- What are the disadvantages of sputtering? Key Challenges and Trade-offs for Thin-Film Deposition



















