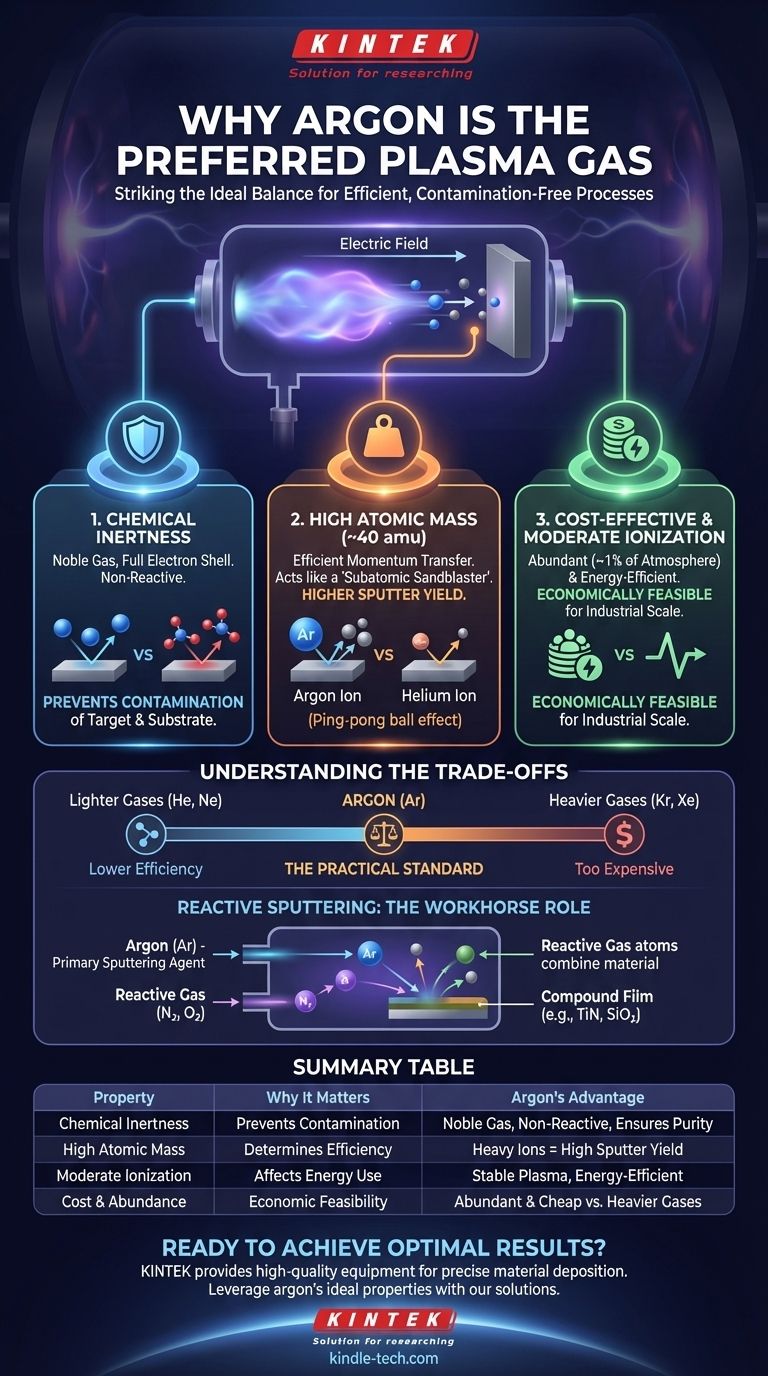
In short, argon is the preferred gas for creating plasma because it strikes a perfect balance between three critical properties: it is chemically inert, it has a high atomic mass, and it is cost-effective. This unique combination makes it highly efficient for physical processes like sputter deposition without causing unwanted chemical reactions that would contaminate the materials.
The choice of argon is not arbitrary; it is a calculated decision based on physics and economics. Its chemical inertness prevents contamination, while its atomic weight provides the physical momentum needed to efficiently eject atoms from a target material, all at a cost that makes industrial-scale processes feasible.

The Ideal Properties of a Plasma Gas
To understand why argon is the industry standard, we must first define what makes a gas suitable for generating a stable, effective plasma for material processing. The ideal gas must satisfy several key requirements.
Chemical Inertness is Paramount
The most crucial property is that the gas does not chemically react with the materials in the vacuum chamber.
Argon is a noble gas, meaning its outermost electron shell is completely full. This makes it extremely stable and non-reactive under most conditions.
In processes like sputter deposition, the goal is to physically transfer a pure material from a source (the target) to a destination (the substrate). If a reactive gas like nitrogen or oxygen were used, it would form unwanted nitrides or oxides on the target and the final film, effectively contaminating the product.
The Critical Role of Atomic Mass
Plasma processes like sputtering are fundamentally physical. Ions from the plasma are accelerated by an electric field and slam into a target material, acting like a subatomic sandblaster.
The effectiveness of this "sandblasting" depends on momentum transfer. Argon, with an atomic mass of approximately 40 amu, is significantly heavier than other common gases like helium (4 amu) or neon (20 amu).
When an argon ion strikes the target, it transfers more kinetic energy per collision, leading to a much higher sputter yield—the number of target atoms ejected per incoming ion. Using a lighter gas like helium would be far less efficient, like trying to knock down bowling pins with a ping-pong ball instead of a bowling ball.
Favorable Ionization Energy
To create a plasma, you must supply enough energy to strip electrons from the gas atoms, a process called ionization. The energy required to do this is the ionization energy.
Argon has a relatively moderate ionization energy. It is low enough that a plasma can be generated and sustained without excessive power consumption, making the process energy-efficient.
While other noble gases have different ionization energies, argon's value represents a practical sweet spot for stable plasma generation in standard equipment.
Understanding the Trade-offs
While argon is the go-to choice, it is not the only option. Understanding its position relative to other gases reveals the economic and technical trade-offs involved.
The Cost Factor: Argon vs. Other Noble Gases
Heavier noble gases like Krypton (Kr) and Xenon (Xe) would actually be even more effective for sputtering due to their higher atomic mass. They would provide a superior sputter yield.
However, these gases are far rarer and, consequently, orders of magnitude more expensive than argon. Argon makes up nearly 1% of Earth's atmosphere, making it abundant and cheap to produce. This makes it the only economically viable choice for most industrial applications.
The Role of Reactive Gases
Sometimes, chemical reactions are desired. In a process called reactive sputtering, a reactive gas like nitrogen (N₂) or oxygen (O₂) is intentionally introduced into the chamber along with the argon.
In this scenario, the argon still does the heavy lifting—its ions are the primary source for sputtering the target material. However, as the sputtered atoms travel to the substrate, they react with the secondary gas to form a specific compound film, such as titanium nitride (TiN) or silicon dioxide (SiO₂). Here, argon acts as the essential, non-interfering "workhorse" plasma gas.
Making the Right Choice for Your Goal
Your choice of gas is dictated entirely by the desired outcome of your plasma process.
- If your primary focus is efficient physical sputtering: Argon offers the best balance of high sputtering yield (due to its mass) and cost-effectiveness.
- If your primary focus is preventing all chemical contamination: Argon's noble gas nature ensures it will not react with your target or substrate, preserving material purity.
- If your primary focus is creating specific compound films: Use argon as the stable base plasma and introduce a secondary reactive gas (like N₂ or O₂) to form the desired chemical compound on your substrate.
Ultimately, argon's widespread use is a testament to its unique and highly practical compromise between ideal physical properties, chemical stability, and economic reality.
Summary Table:
| Property | Why It Matters for Plasma | Argon's Advantage |
|---|---|---|
| Chemical Inertness | Prevents contamination of target and substrate materials. | As a noble gas, argon is non-reactive, ensuring material purity. |
| High Atomic Mass (~40 amu) | Determines sputtering efficiency via momentum transfer. | Heavy ions efficiently eject target atoms, leading to a high sputter yield. |
| Moderate Ionization Energy | Affects the energy required to create and sustain the plasma. | Allows for stable plasma generation without excessive power consumption. |
| Cost & Abundance | Makes industrial-scale processes economically feasible. | Abundant in the atmosphere, making it far cheaper than heavier noble gases. |
Ready to achieve optimal results in your lab's sputtering or plasma processes?
At KINTEK, we specialize in providing the high-quality lab equipment and consumables you need for precise material deposition. Our expertise ensures you get the right tools to leverage argon's ideal properties for contamination-free, efficient results.
Contact our experts today to discuss how our solutions can enhance your laboratory's capabilities and drive your research forward.
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- Why does PECVD commonly use RF power input? For Precise Low-Temperature Thin Film Deposition
- What is plasma enhanced? A Guide to Low-Temperature, High-Precision Manufacturing
- What is plasma enhanced chemical vapor deposition PECVD equipment? A Guide to Low-Temperature Thin Film Deposition
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings



















