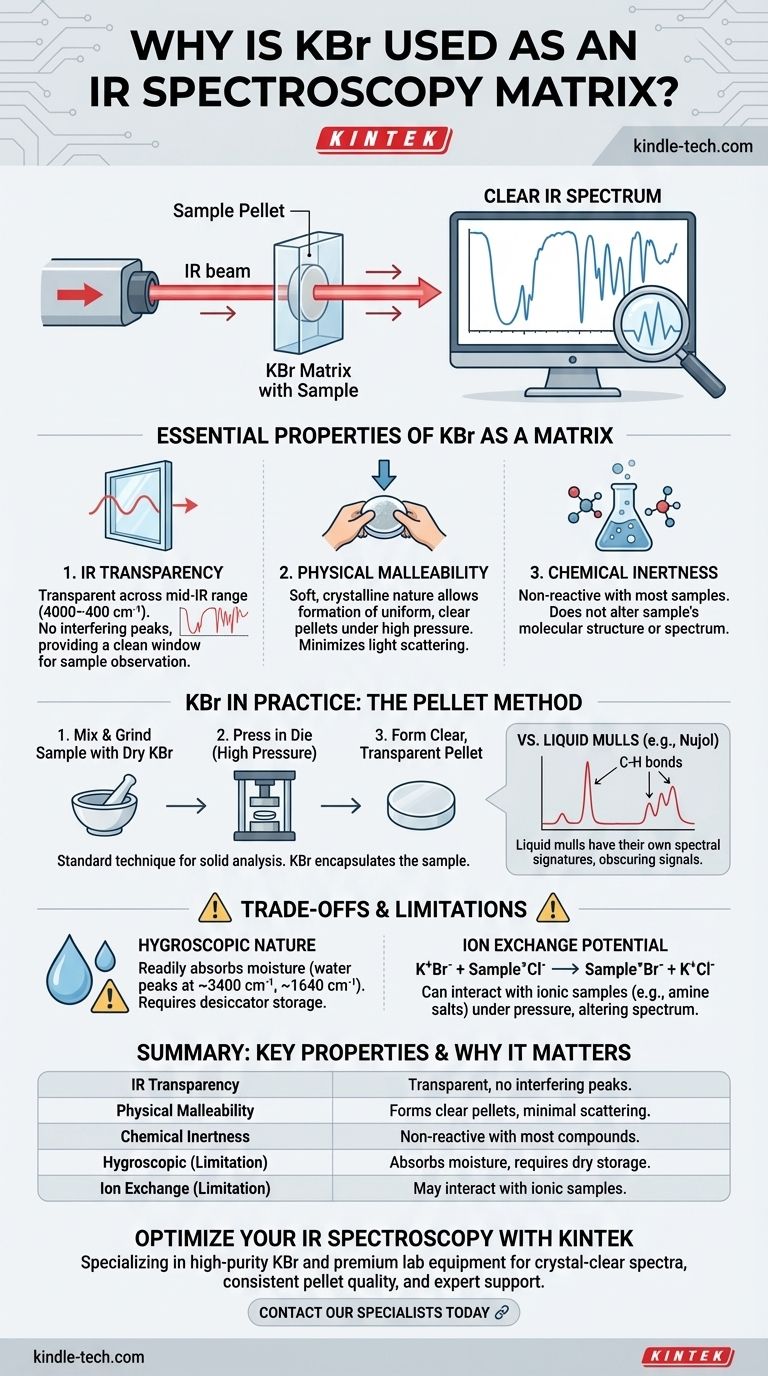
To be direct, Potassium Bromide (KBr) is used as a matrix for infrared (IR) spectroscopy because it is transparent to IR radiation and physically soft. Its transparency ensures that it doesn't produce its own spectral signals that would obscure the sample's, while its soft, crystalline nature allows it to be pressed under high pressure into a uniform, glass-like pellet that holds the sample for analysis.
The core challenge in transmission IR spectroscopy is to suspend a solid sample in a medium that is effectively "invisible" to the infrared beam. KBr is the industry standard for this task because it meets the essential criteria of being transparent, non-reactive, and physically malleable, allowing for clear and accurate sample measurement across the most useful portion of the IR spectrum.

The Essential Properties of an IR Matrix
To understand why KBr is so prevalent, we must first define the ideal characteristics of a matrix material used for embedding solid samples in IR spectroscopy. The material must not interfere with the analysis.
Infrared Transparency
The single most important property is that the matrix material does not absorb infrared light in the region of interest.
KBr is transparent across the entire mid-infrared range (4000 cm⁻¹ to 400 cm⁻¹), which is where the vast majority of characteristic molecular vibrations occur. This means it provides a clean, clear window through which to observe the sample's unique absorption spectrum without interference.
Physical Malleability and Softness
Solid samples must be finely ground and evenly dispersed to minimize the scattering of infrared light, which can distort the spectrum (an issue known as the Christiansen effect).
KBr is a relatively soft, alkali halide salt. When ground with a sample and subjected to high pressure (several tons), its crystalline structure deforms and flows, encapsulating the sample particles. This process forms a solid, semi-transparent pellet that is ideal for analysis.
Chemical Inertness
The matrix material should not react with the sample. Any chemical reaction would alter the sample's molecular structure, and the resulting spectrum would not be representative of the original material.
For most organic and many inorganic compounds, KBr is chemically inert and serves as a passive suspension medium.
KBr in Practice: The Pellet Method
While you asked about its use as a "mulling agent," KBr is most famously used to create solid pellets. The term "mull" typically refers to grinding a solid with a liquid (like Nujol oil) to form a paste.
The KBr Pellet Method
This is the standard technique. A small amount of the solid sample (around 1%) is intimately ground with high-purity, dry KBr powder. This mixture is then pressed in a die under immense pressure to form a thin, transparent disc or pellet.
The quality of the pellet is critical. A good pellet is clear and uniform, allowing the IR beam to pass through with minimal scattering, resulting in a clean spectrum.
Distinguishing from Liquid Mulls
Liquid mulling agents like Nujol (mineral oil) or Fluorolube are also used. However, they have their own spectral signatures. Nujol consists of C-H bonds and will show strong absorptions in those regions, obscuring the sample's signal.
KBr's primary advantage over liquid mulls is its complete lack of interfering peaks across the mid-IR range.
Understanding the Trade-offs and Limitations
Despite being the standard, KBr is not without its challenges. Understanding these is crucial for accurate analysis.
The Critical Problem of Water
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has a very strong, broad IR absorption band around 3400 cm⁻¹ (O-H stretching) and a sharp peak around 1640 cm⁻¹ (H-O-H bending).
If the KBr used is not perfectly dry, these water peaks will appear in your spectrum, potentially obscuring important sample features. For this reason, spectroscopy-grade KBr must always be stored in a desiccator or drying oven.
Potential for Ion Exchange
Because KBr is an ionic salt (K⁺Br⁻), it can sometimes interact with ionic samples. A classic example is the analysis of hydrochloride salts of amines (R-NH₃⁺Cl⁻).
In the high-pressure environment of the pellet, the bromide ion (Br⁻) from the matrix can exchange with the chloride ion (Cl⁻) of the sample. This alters the sample and changes its spectrum, leading to incorrect interpretation.
Pressure-Induced Effects
The high pressure used to form the pellet can sometimes induce changes in the sample's crystalline form (polymorphism). This can lead to slight shifts or changes in the resulting spectrum compared to the sample's native state.
Making the Right Choice for Your Goal
Selecting the correct sample preparation method depends entirely on your sample's properties and your analytical goals.
- If your primary focus is routine mid-IR analysis of a stable, non-ionic compound: The KBr pellet method is the gold standard for its clarity, low cost, and broad spectral window. Always ensure your KBr is impeccably dry.
- If your sample is sensitive to moisture or potentially reactive with KBr: A liquid mull (like Nujol) is a better choice, as long as you can tolerate its interference peaks in the C-H regions.
- If your analysis extends into the Far-IR region (below 400 cm⁻¹): KBr is not suitable as it begins to absorb light. You must use a different matrix, such as Cesium Iodide (CsI) or pressed polyethylene.
Ultimately, understanding these principles ensures your sample preparation enhances, rather than compromises, the accuracy of your spectroscopic results.
Summary Table:
| Property | Why It Matters for IR Spectroscopy |
|---|---|
| IR Transparency | Transparent across mid-IR range (4000-400 cm⁻¹) - no interfering peaks |
| Physical Malleability | Forms clear pellets under pressure with minimal light scattering |
| Chemical Inertness | Non-reactive with most organic and inorganic compounds |
| Limitation: Hygroscopic | Absorbs moisture - requires dry storage to avoid water peaks |
| Limitation: Ion Exchange | May interact with ionic samples like hydrochloride salts |
Optimize Your IR Spectroscopy with KINTEK's Premium Lab Equipment
Are you struggling with sample preparation for infrared spectroscopy? KINTEK specializes in high-purity KBr and laboratory equipment designed specifically for analytical chemistry applications. Our spectroscopy-grade materials ensure:
• Crystal-clear IR spectra with minimal interference • Consistent pellet quality for reproducible results • Moisture-controlled packaging to prevent hygroscopic issues • Expert technical support for your specific analytical challenges
Whether you're working with organic compounds, pharmaceuticals, or materials research, KINTEK provides the reliable consumables and equipment your laboratory needs. Contact our specialists today to discuss how we can enhance your IR spectroscopy workflow and deliver superior analytical outcomes.
Visual Guide
Related Products
- kbr pellet press 2t
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- XRF & KBR plastic ring lab Powder Pellet Pressing Mold for FTIR
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What is the difference between plate and frame and recessed chamber filter press? Flexibility vs. Efficiency
- What is the function of a laboratory hydraulic pressing device in U-bend SCC tests? Master Stress Corrosion Cracking
- What is the purpose of using a laboratory hydraulic press for cold-press pre-forming? Achieve Optimal Sintering Density
- What are the steps involved in sample preparation? A Guide to Accurate and Reliable Analysis
- How does a laboratory hydraulic press ensure sample quality for Cerium Oxide pellets? Achieving Precision Geometry
- What is the maximum capacity of a hydraulic press machine? From 20 to 80,000+ Tons
- Why is a powder hydraulic press used to create reaction pellets during the carbothermic reduction of magnesium?
- What are the advantages of a power press machine? Achieve High-Speed, Cost-Effective Metal Stamping



















