In short, Potassium bromide (KBr) is used because it is transparent to infrared light and becomes plastic under pressure. This unique combination of properties allows it to form a solid, glass-like disc that holds a powdered sample in the path of the IR beam without producing its own interfering spectral signals. It effectively acts as a solid, non-reactive solvent for IR analysis.
The core challenge in analyzing solid samples with IR spectroscopy is getting them into a form that infrared light can pass through uniformly. KBr solves this by serving as an ideal matrix material—it is invisible to the IR spectrometer and can be pressed into a transparent pellet, making it the industry standard for this technique.

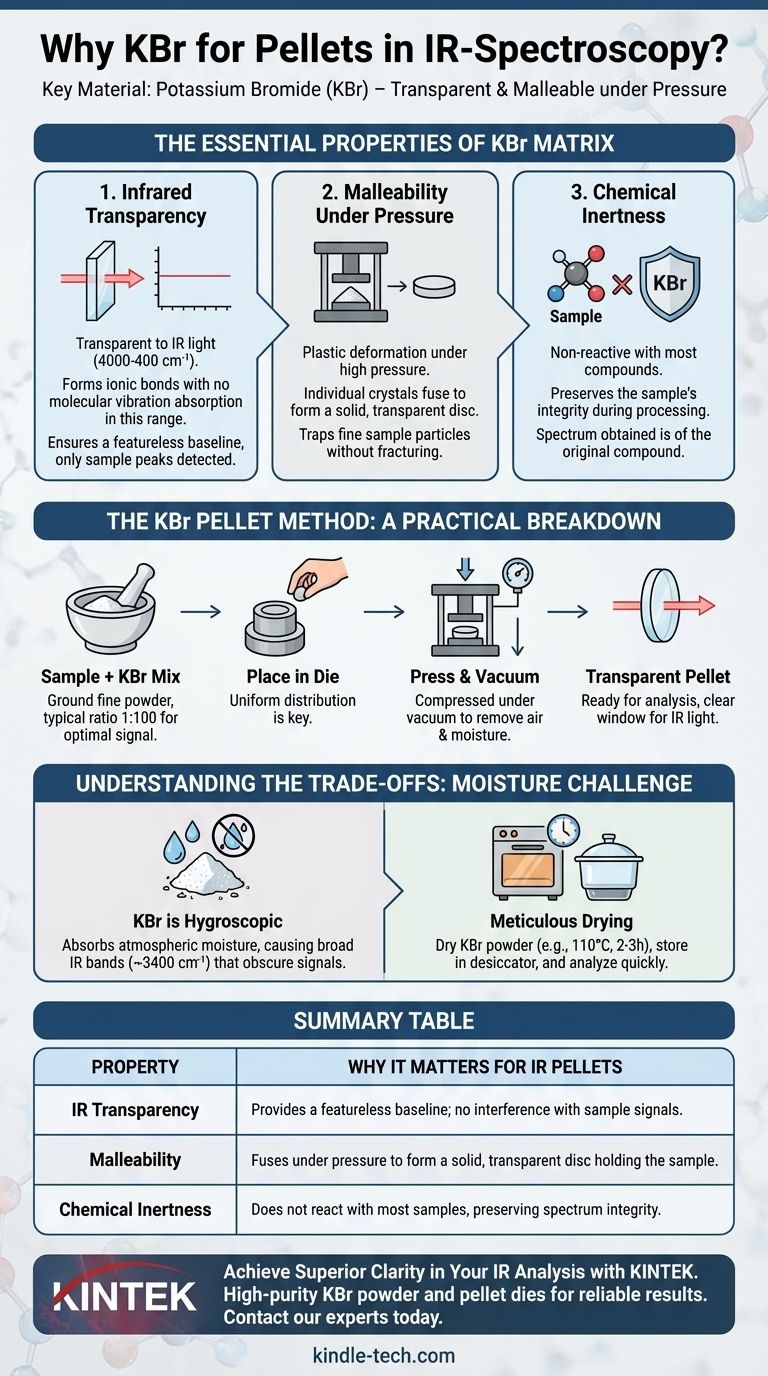
The Essential Properties of a Pellet Matrix
To understand why KBr is the default choice, it's helpful to first define the ideal characteristics of a material used to hold a solid sample for IR analysis. The matrix material must not interfere with the measurement in any way.
Infrared Transparency
The single most important property is transparency across the mid-infrared region (typically 4000-400 cm⁻¹). KBr is an alkali halide, a type of ionic salt that does not have molecular bonds that vibrate and absorb energy in this specific range.
This means a pure KBr pellet will produce a flat, featureless baseline, ensuring that any absorption peaks detected are from your sample, not the matrix holding it.
Malleability Under Pressure
KBr powder, when subjected to high pressure (typically several tons) in a die, exhibits plastic deformation. The individual salt crystals fuse together to form a solid, transparent, or translucent sheet.
This property is critical because it allows a fine powder mixture to become a solid disc without fracturing, effectively trapping the finely dispersed sample particles in a uniform, solid-state solution.
Chemical Inertness
For the vast majority of organic and inorganic compounds, KBr is chemically inert. It does not react with the sample during the grinding or pressing process. This ensures the spectrum you obtain is of the original compound, not some new substance formed by a reaction with the matrix.
The KBr Pellet Method: A Practical Breakdown
The properties of KBr directly inform the standard procedure for preparing a sample. Each step is designed to leverage these characteristics for a high-quality measurement.
Sample Preparation and Ratios
The sample is first ground to a fine powder and mixed with high-purity, dry KBr powder. A typical ratio is about 1 part sample to 100 parts KBr.
This dilution ensures that the sample's absorption peaks are within the detector's optimal range and not overly saturated. The fine grinding is essential for uniform distribution and minimizing light scattering.
The Role of Pressure and Vacuum
The mixture is placed into a pellet die and compressed with a hydraulic press. This process is almost always performed under vacuum.
The vacuum serves two purposes: it removes trapped air that can cause the pellet to be opaque or fracture, and more importantly, it helps remove any adsorbed moisture (water) from the highly hygroscopic KBr.
The Importance of a "Blank" Pellet
Before analyzing the sample pellet, a background spectrum is run using a KBr-only pellet (a "blank") or an empty pellet holder.
The instrument's software then automatically subtracts this background from the sample's spectrum. This removes any minor absorption signals from residual moisture or CO₂ in the spectrometer, as well as correcting for light-scattering effects from the pellet itself.
Understanding the Trade-offs and Common Pitfalls
While KBr is the standard, achieving good results requires careful technique. Its primary drawback is its affinity for water.
The Problem of Moisture
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong, broad absorption bands in the IR spectrum (~3400 cm⁻¹) which can easily obscure important signals from the sample.
To combat this, the KBr powder must be thoroughly dried in an oven (e.g., at 110 °C for 2-3 hours) before use and stored in a desiccator. The pellet should also be prepared and analyzed quickly.
Risk of Inconsistent Sample Distribution
If the sample and KBr are not pulverized to a very fine powder and mixed thoroughly, the sample will not be distributed evenly in the pellet. This leads to a sloping baseline and distorted, inaccurate peak shapes due to light scattering (the Christiansen effect).
Potential for Ionic Exchange
In rare cases with certain salt samples (e.g., hydrochloride salts of amines), an ion exchange can occur between the sample and the KBr matrix. This can alter the sample's spectrum, a possibility the analyst must consider if the results are unexpected.
Making the Right Choice for Your Goal
Proper KBr pellet technique is a cornerstone of reliable IR analysis for solid samples. Your specific focus will determine which step to prioritize.
- If your primary focus is accuracy: Meticulously dry your KBr powder and run a high-quality background spectrum with a pure KBr blank to ensure you are only measuring your sample.
- If your primary focus is signal clarity: Prioritize extremely fine grinding and thorough mixing of the sample and KBr to minimize light scattering and achieve a flat baseline.
- If you suspect sample degradation: Be gentle with grinding and consider if another method, like a Nujol mull, is more appropriate if your compound is pressure- or heat-sensitive.
Ultimately, mastering the KBr pellet method is about controlling variables to ensure the material serves its purpose as an invisible window to your sample's molecular structure.
Summary Table:
| Property | Why It Matters for IR Pellets |
|---|---|
| IR Transparency | Provides a featureless baseline; doesn't interfere with sample signals. |
| Malleability | Fuses under pressure to form a solid, transparent disc that holds the sample. |
| Chemical Inertness | Does not react with most samples, preserving the integrity of the spectrum. |
Achieve superior clarity in your IR spectroscopy analysis. The KBr pellet method is fundamental for accurate solid sample characterization. KINTEK specializes in providing the high-purity lab equipment and consumables—including KBr powder and pellet dies—that your laboratory needs for reliable results. Contact our experts today to discuss your specific application and ensure your lab is equipped for success.
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What is the function of an industrial hydraulic press used with steel dies? Achieve High-Density CrFeCuMnNi Compacts
- Why use a laboratory hydraulic press for sulfide batteries? Achieve 445 MPa for Optimal Ionic Conductivity
- Does hydraulic pressure change with temperature? Understanding the Critical Link for System Safety
- How do you increase the speed of a hydraulic press? Boost Cycle Times & Productivity
- What is the pressing process of ceramics? A Guide to Precise, High-Strength Manufacturing
- What are the applications of laboratory hydraulic presses in phase-transfer catalytic desulfurization research? Achieve Precise Catalyst Pelletization
- Why is a laboratory hydraulic press used to compress powders into pellets? Enhance Solid-State Reaction Kinetics
- What elements can handheld XRF detect? From Magnesium to Uranium, Understand Its Capabilities and Limits



















