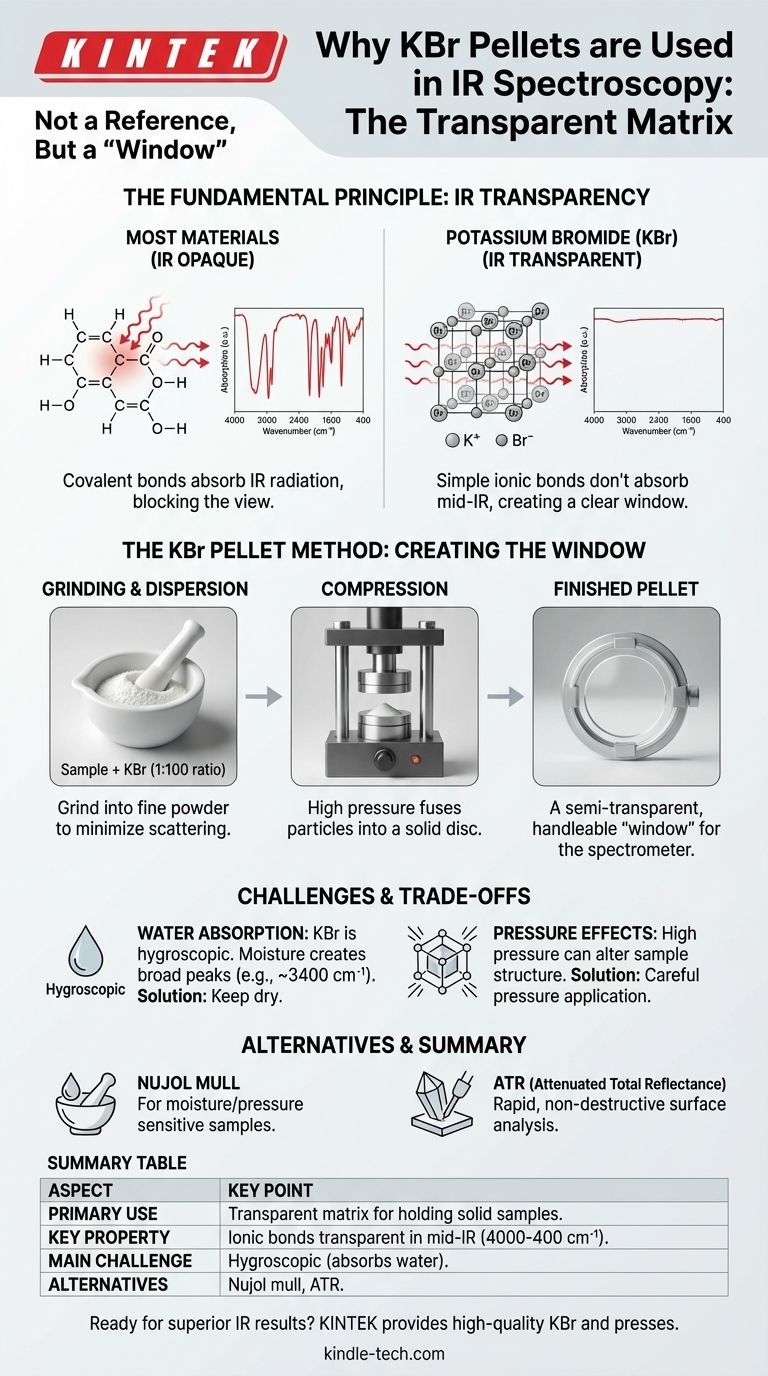
Potassium bromide (KBr) is not used as a reference, but rather as a transparent matrix to hold a solid sample for analysis. In infrared (IR) spectroscopy, the goal is to measure how a sample absorbs IR radiation, so the material used to prepare and hold that sample must be invisible to the spectrometer. KBr is chosen because its simple ionic bonds do not absorb light in the mid-infrared region, creating a clear "window" through which the spectrum of the sample can be measured without interference.
The core challenge in analyzing solid samples with IR spectroscopy is isolating the sample's spectrum from its surroundings. KBr is used as a sample preparation medium because it is transparent to infrared light, allowing the spectrometer to "see" only the sample, not the material holding it.

The Fundamental Principle: IR Transparency
To understand why KBr is the standard, we must first understand why most materials are not suitable for holding an IR sample.
Why Most Materials Block Infrared Light
Infrared spectroscopy works by measuring the absorption of IR energy, which causes the chemical bonds within a molecule to vibrate.
Nearly all organic and many inorganic compounds are built with covalent bonds (e.g., C-H, O-H, C=O). These bonds absorb IR radiation at specific frequencies, creating the unique spectral fingerprint we use for identification.
Because most materials have these types of bonds, they are opaque to IR radiation, making them unsuitable as a sample holder.
The Unique Property of KBr
KBr is an alkali halide salt. It consists of potassium (K⁺) and bromide (Br⁻) ions held together in a rigid crystal lattice by ionic bonds.
The vibrations of these simple, strong ionic bonds occur at very low frequencies. These frequencies fall into the far-infrared region (typically below 400 cm⁻¹), well outside the mid-infrared range (4000–400 cm⁻¹) used for most chemical analysis.
KBr as an "IR Window"
Because it does not absorb light in the crucial mid-IR range, KBr acts as a perfect window. It allows the spectrometer's beam to pass through it unimpeded, interact with the dispersed sample molecules, and reach the detector.
This ensures that the resulting spectrum is purely a result of your sample, not a combination of your sample and the matrix material.
The Practical Need: Preparing a Solid Sample
Simply placing a solid chunk in the spectrometer is ineffective. The beam cannot pass through it, and light scattering from a rough surface creates significant noise. The KBr pellet method solves this.
The Goal: Uniform Dispersion
To get a clean spectrum, the sample must be diluted and dispersed evenly. A common ratio is approximately 1 part sample to 100 parts KBr.
This dilution prevents the sample's own absorption from being too strong (saturating the detector) and ensures the IR beam interacts with a representative amount of the substance.
Creating an Optically Clear Disc
The sample and KBr powder are first ground together into an extremely fine powder. This minimizes an optical artifact known as scattering, which can distort the spectrum.
The fine powder mixture is then placed in a die and compressed with a hydraulic press. This high pressure fuses the KBr particles into a solid, semi-transparent disc, which is easy to handle and mount in the spectrometer's sample holder.
Understanding the Trade-offs and Pitfalls
While KBr is the industry standard, it is not without its challenges. Proper technique is critical for obtaining a high-quality spectrum.
The Primary Challenge: Water Contamination
KBr is hygroscopic, meaning it readily absorbs moisture from the atmosphere. Water has very strong and broad IR absorption bands (a wide peak around 3400 cm⁻¹ and a sharp peak near 1640 cm⁻¹).
If your KBr is "wet," these water peaks will appear in your spectrum and can easily obscure important peaks from your actual sample. To avoid this, KBr powder must be stored in a desiccator and can be dried in an oven before use.
The Effect of Pressure
The high pressure used to form the pellet can sometimes induce changes in the crystalline structure of the sample (polymorphism). This can lead to slight shifts or changes in the IR spectrum.
Potential for Ion Exchange
In rare cases, the bromide ion (Br⁻) from the KBr matrix can react with certain types of samples, such as salts containing a different halide. This exchange can create a new compound, and you end up measuring a spectrum of the reaction product, not your original sample.
Making the Right Choice for Your Goal
While the KBr pellet is a powerful and common technique, it is one of several methods available for solid-state IR analysis.
- If your primary focus is high-resolution analysis of a stable solid: The KBr pellet method, when performed carefully to exclude moisture, provides excellent, high-quality spectra.
- If your sample is sensitive to moisture or pressure: Consider using a Nujol mull, where the sample is ground into a mineral oil paste, which is then smeared between two salt plates.
- If your goal is rapid, non-destructive analysis: Attenuated Total Reflectance (ATR) spectroscopy is a modern alternative that requires minimal sample preparation and analyzes the surface of a material directly.
Understanding the properties of your sample and the goal of your analysis is the key to acquiring a clean, accurate, and meaningful IR spectrum.
Summary Table:
| Aspect | Key Point |
|---|---|
| Primary Use | Transparent matrix for holding solid samples, not a reference. |
| Key Property | Ionic bonds are transparent in the mid-IR region (4000-400 cm⁻¹). |
| Main Advantage | Creates a clear "window" to measure only the sample's spectrum. |
| Main Challenge | Hygroscopic; can absorb water, which contaminates the spectrum. |
| Alternative Methods | Nujol mull (moisture-sensitive samples), ATR (rapid analysis). |
Ready to achieve superior results in your IR spectroscopy?
KINTEK specializes in providing the high-quality lab equipment and consumables you need for precise sample preparation, including reliable KBr powder and hydraulic presses for creating perfect pellets. Our expertise ensures you can minimize interference and obtain clean, accurate spectra for your analysis.
Contact our experts today to discuss how we can support your laboratory's specific needs and help you choose the right tools for your spectroscopy workflow.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- kbr pellet press 2t
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- What materials are used in electron beam evaporation? Master High-Purity Thin Film Deposition
- Does sintering increase conductivity? Yes, by transforming powders into dense, conductive solids.
- What is the future potential for biomass? Unlocking a Sustainable Bioeconomy
- What are some common applications of ultra-low temperature freezers? Essential for Biomedical, Pharma, and Clinical Storage
- What is direct current magnetron sputtering? A Guide to High-Quality Thin Film Deposition
- What is a rotary vacuum evaporator? A Guide to Gentle & Efficient Solvent Removal
- What is the highest temperature of a furnace? Unlocking the Limits of Extreme Heat
- Why are vacuum drying and argon purging equipment necessary for SILP catalyst impregnation? Optimize Performance Now



















