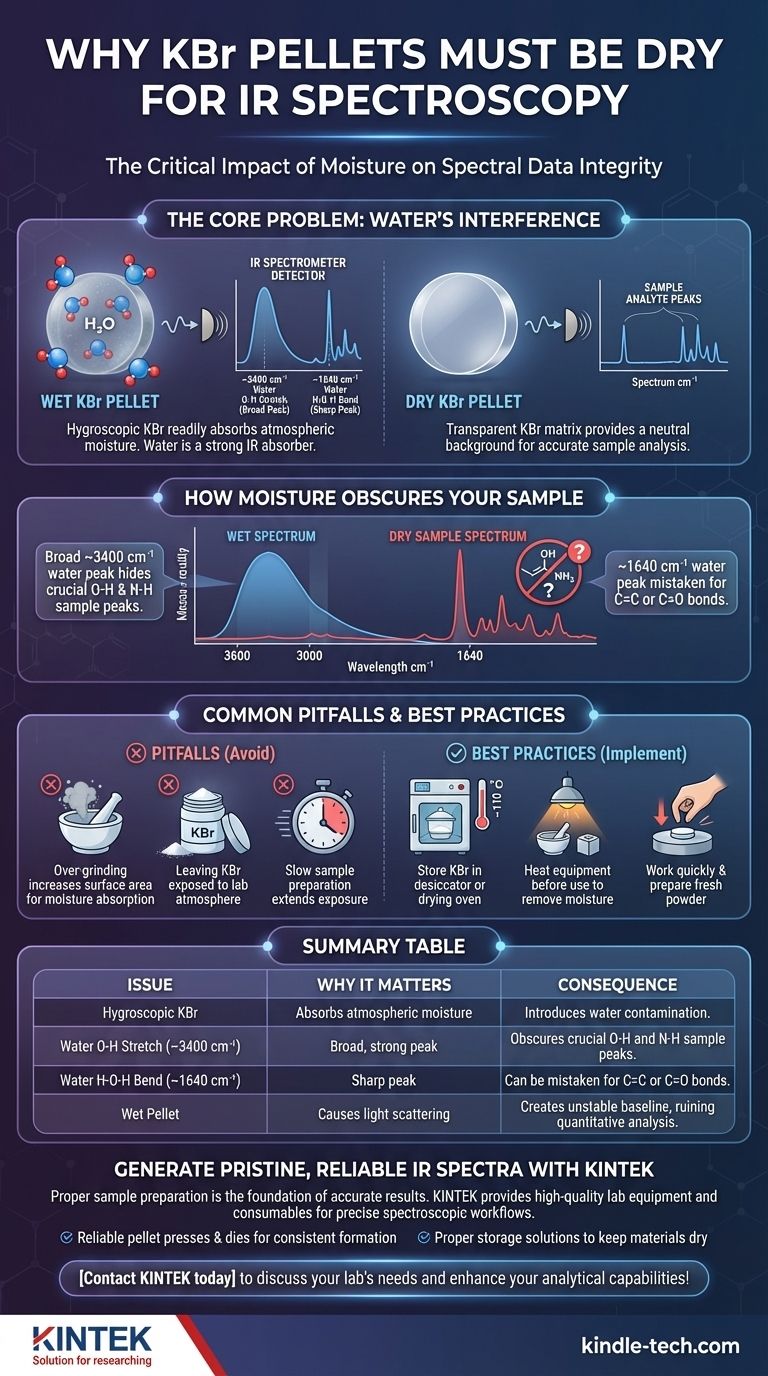
In short, potassium bromide (KBr) for an infrared (IR) spectroscopy pellet must be scrupulously dry because any absorbed water will produce strong, interfering signals in your spectrum. This contamination can obscure or be mistaken for features of your actual sample, leading to incorrect analysis and unusable data.
The core issue is that KBr is hygroscopic, meaning it readily absorbs atmospheric moisture. Because water (H₂O) is a very strong IR absorber, its presence creates large, unwanted peaks in the spectrum that compromise the integrity of your results.

The Core Problem: Water's Interference in IR Spectroscopy
To understand why dryness is critical, you must first understand how water behaves in an IR spectrometer. The goal is to obtain a clean spectrum of your analyte, not a combined spectrum of your analyte and water.
Why Water is a Problem
The chemical bonds within a water molecule, specifically the oxygen-hydrogen (O-H) bonds, are excellent absorbers of infrared radiation. When IR radiation of a specific frequency hits these bonds, they vibrate, and that energy absorption is registered by the detector as a signal (a "peak").
Because KBr itself is transparent to IR radiation in the typical analysis range (4000-400 cm⁻¹), it is used as a neutral matrix to hold the sample. Any contamination within this matrix, especially a strong absorber like water, will show up clearly.
The Signature of Water Contamination
Water contamination is easily identifiable by two characteristic absorptions in your IR spectrum:
- A very broad, strong peak centered around 3400 cm⁻¹. This is due to the O-H stretching vibrations.
- A weaker, but still significant, sharp peak around 1640 cm⁻¹. This is from the H-O-H bending vibration.
The presence of these two signals is a definitive sign that your KBr pellet is wet.
How Moisture Obscures Your Sample
The strong, broad water peak around 3400 cm⁻¹ is particularly problematic. This region of the spectrum is also where crucial functional groups like alcohols (O-H) and amines (N-H) from your actual sample absorb.
If your KBr is wet, the massive water peak can completely overlap and hide these important sample peaks, making it impossible to confirm their presence or absence.
Common Pitfalls and Best Practices
Avoiding moisture contamination requires understanding the properties of KBr and adhering to strict handling procedures. Simply being aware of the risk is the first step.
KBr is Naturally Hygroscopic
The fundamental challenge is that potassium bromide is a hygroscopic salt. It naturally attracts and absorbs water molecules from the ambient air. Leaving KBr powder exposed to the lab atmosphere for even a short time is enough to introduce significant contamination.
The Grinding Fallacy
A common mistake is to grind KBr powder extensively to make it finer, believing this will improve the pellet. While a fine powder is necessary, over-grinding significantly increases the surface area of the KBr crystals.
This newly exposed surface area is highly active and rapidly absorbs moisture from the air. It is far better to prepare fresh powder immediately before use.
Preparing a Dry, High-Quality Pellet
The reference techniques are industry standards for a reason. They directly combat the hygroscopic nature of KBr.
- Proper Storage: Always store KBr powder in a tightly sealed container inside a desiccator or a low-temperature drying oven (~110 °C). This keeps it in a moisture-free environment.
- Dry Equipment: Gently heat the mortar, pestle, and die set under a heat lamp or in an oven before use. This drives off any condensed moisture on the metal surfaces.
- Work Quickly: Prepare your sample and press the pellet efficiently to minimize the powder's exposure time to the lab environment.
- Create Fresh Powder: If you suspect your bulk KBr powder has been exposed to moisture, do not grind the existing powder. Instead, take a few larger crystals or chunks of KBr and grind them to create fresh, dry powder for immediate use.
Making the Right Choice for Your Analysis
Ultimately, the quality of your preparation technique directly determines the quality of your spectroscopic data. There is no shortcut around the need for dry KBr.
- If your primary focus is accurate sample identification: Dry KBr is non-negotiable. You cannot risk misinterpreting a broad water peak at 3400 cm⁻¹ as evidence of an alcohol or mistaking the 1640 cm⁻¹ peak for a C=C or C=O bond.
- If your primary focus is quantitative analysis: A wet pellet will cause light scattering and a sloping, unstable baseline, making it impossible to perform the background subtractions necessary for accurate measurements.
- If your primary focus is troubleshooting a bad spectrum: The first step should always be to check for the classic signs of water contamination. A clean spectrum starts with clean, dry materials.
Adhering to proper sample preparation is the foundational skill for generating reliable and interpretable IR spectra.
Summary Table:
| Issue | Why It Matters | Consequence |
|---|---|---|
| Hygroscopic KBr | Absorbs atmospheric moisture | Introduces water contamination |
| Water O-H Stretch (~3400 cm⁻¹) | Broad, strong peak | Obscures crucial O-H and N-H sample peaks |
| Water H-O-H Bend (~1640 cm⁻¹) | Sharp peak | Can be mistaken for C=C or C=O bonds |
| Wet Pellet | Causes light scattering | Creates unstable baseline, ruining quantitative analysis |
Generate Pristine, Reliable IR Spectra with KINTEK
Don't let moisture contamination compromise your analysis. Proper sample preparation is the foundation of accurate results. KINTEK specializes in high-quality lab equipment and consumables designed to support precise spectroscopic workflows.
We help you ensure accuracy by providing:
- Reliable pellet presses and dies for consistent pellet formation.
- Proper storage solutions to keep your materials dry.
Let our expertise in laboratory supplies help you achieve clean, interpretable data every time.
Contact KINTEK today to discuss your lab's needs and enhance your analytical capabilities!
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- kbr pellet press 2t
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
People Also Ask
- What are the specific conditions and steps in HPHT diamond growth? Master the Art of Synthetic Diamond Production
- What equipment is needed for XRF analysis? A Guide to the Essential Tools for Accurate Results
- Why is a 350 MPa laboratory hydraulic press necessary for sulfide solid-state electrolytes? Achieve peak density.
- What role does a laboratory hydraulic press play in testing conductivity? Enhancing Nanoparticle Powder Analysis
- Why is 10 MPa pressure necessary for all-solid-state lithium coin cells? Enhance Interfacial Contact and Performance
- Is hydraulic press safe? Ensure Operator Safety with Proper Training and Protocols
- How many types of XRF instruments are there? A Guide to EDXRF vs. WDXRF
- What role does a laboratory hydraulic press play in preparing LixScCl3+x samples for EIS? Achieve Reliable Conductivity



















