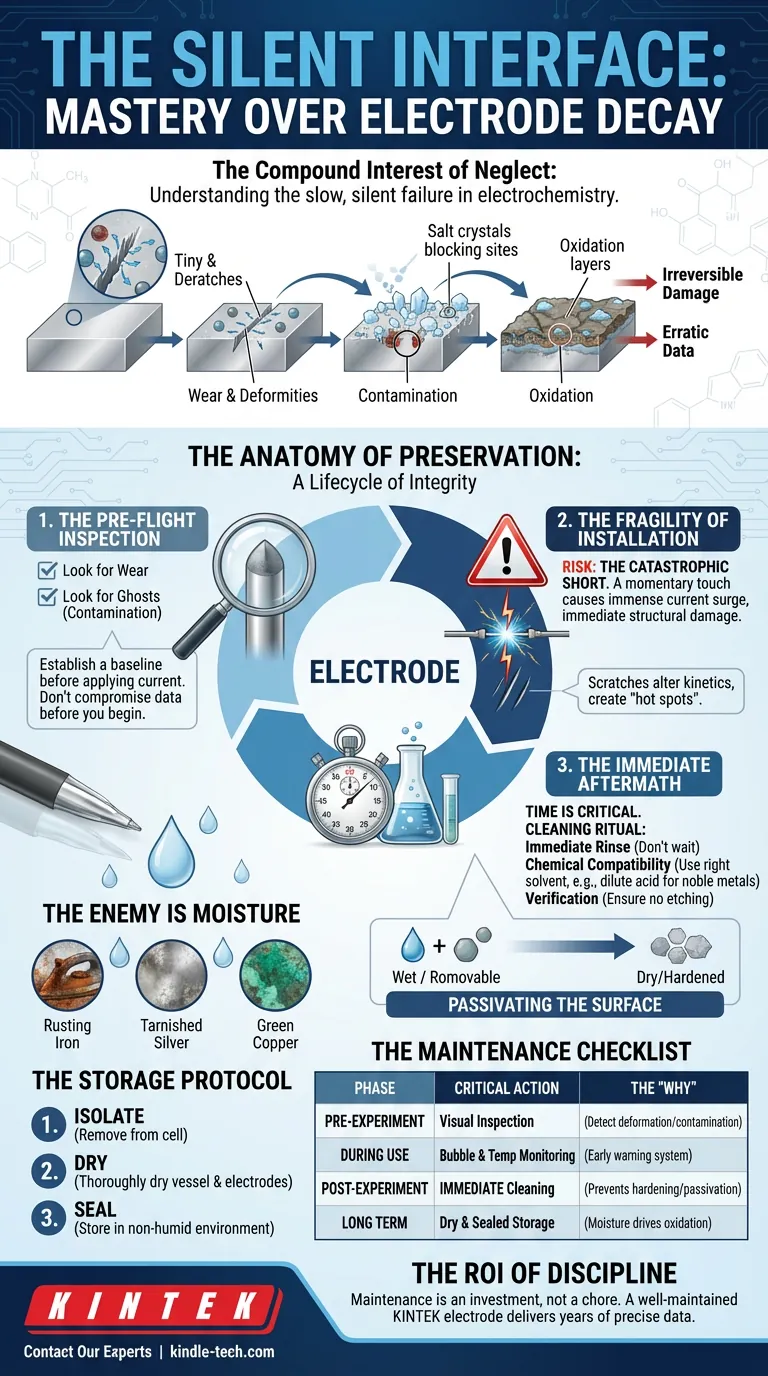
The Compound Interest of Neglect
In the laboratory, entropy is undefeated.
We often think of equipment failure as a dramatic event—a shattered beaker or a blown fuse. But in electrochemistry, failure is usually silent. It is a slow, creeping accumulation of microscopic errors.
An electrode does not stop working all at once. It fades.
A scratch here disrupts current distribution. A drying salt crystal there blocks an active site. Oxidation slowly reclaims the metal. By the time you notice the data is erratic, the damage is already irreversible.
The difference between a reliable experiment and a waste of resources is not luck. It is the discipline of maintenance.
The Anatomy of Preservation
The electrode is the interface between the physical and the chemical. It is a precision instrument disguised as a piece of metal.
Treating it with "care" is too vague. You need a system. A protocol reduces the cognitive load of the operator and ensures that the physics of the reaction remain constant.
Here is the lifecycle of electrode integrity.
1. The Pre-Flight Inspection
Before a pilot takes off, they walk around the plane. They are looking for anomalies. You must do the same.
Before applying current, inspect the surface. You are establishing a baseline.
- Look for wear: Are there physical deformities?
- Look for ghosts: Is there contamination from the last experiment?
If the surface is compromised before you begin, your data is compromised before you record it.
2. The Fragility of Installation
There is a specific moment of high risk: the installation.
Electrodes are often physically robust but electrochemically sensitive. Scratches alter reaction kinetics. They create "hot spots"—localized areas where current flows unevenly.
The Catastrophic Short: The greatest danger is the short circuit. A momentary touch between electrodes during setup generates an immense current surge. This isn't just a spark; it is immediate, irreversible structural damage to the cell.
3. The Immediate Aftermath
The most critical variable in cleaning is time.
When an experiment ends, the clock starts. Reaction products are wet and removable. Give them an hour, and they dry. Give them a day, and they harden.
The residue effectively "passivates" the surface. It builds a wall between the electrode and the electrolyte.
The Cleaning Ritual:
- Immediate Rinse: Do not wait.
- Chemical Compatibility: Use the right solvent. For noble metals like platinum, a dilute acid soak (e.g., 1M nitric acid) followed by deionized water is standard.
- Verification: Ensure the cleaning agent itself doesn't etch the material.
The Enemy is Moisture
Iron rusts. Silver tarnishes. Copper turns green.
Nature is constantly trying to return refined metals to their oxide states. Water is the catalyst for this process.
If you store an electrode wet, you are inviting corrosion. If you store it in a humid room, you are accepting slow degradation.
The Storage Protocol:
- Isolate: Pour the electrolyte into a sealed container. Never leave it in the cell.
- Dry: Thoroughly dry the vessel and the electrodes.
- Seal: Store components in a non-humid environment.
Summary: The Maintenance Checklist
The goal is not just cleanliness; it is consistency. By standardizing your care, you remove variables from your data.
| Phase | Critical Action | The "Why" |
|---|---|---|
| Pre-Experiment | Visual Inspection | To detect deformation or "ghost" contamination. |
| During Use | Bubble & Temp Monitoring | Early warning system for reaction failure. |
| Post-Experiment | Immediate Cleaning | Prevents residue from hardening and passivating the surface. |
| Long Term | Dry & Sealed Storage | Moisture is the primary driver of oxidation. |
The ROI of Discipline
Maintenance is often viewed as a chore—the tax we pay for doing science.
In reality, it is an investment. A well-maintained electrode from KINTEK can deliver years of precise data. A neglected one will become a variable you cannot control.
We build our equipment to withstand the rigors of the laboratory, but we rely on your discipline to keep them singing.
If you are looking for equipment that rewards this level of precision—or if you need advice on the chemical compatibility of your current setup—we are here to help.
Visual Guide
Related Products
- Electrode Polishing Material for Electrochemical Experiments
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Platinum Sheet Electrode for Battery Lab Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications