Evaporation Plating
Definition and Principle
Evaporation coating is a process where a substance is heated to a point where it evaporates, and the resulting vapor condenses on a solid surface, forming a thin film. This technique involves placing the material to be evaporated—such as metals or compounds—into a mo crucible or suspending it on a tungsten coil, which serves as the evaporation source. The workpieces that require coating are positioned in front of the crucible.
Prior to heating, the system is pumped to achieve a high vacuum state, which is crucial for the process. Once the vacuum is established, the crucible is heated, causing the material to evaporate. The atoms or molecules of the evaporated material then travel through the vacuum and condense on the surface of the substrate, forming a thin, uniform layer. This method ensures that the coating adheres well to the substrate due to the controlled environment and the direct deposition of the material.
The principle behind evaporation coating is based on the physical phenomenon of evaporation and condensation. By maintaining a high vacuum, the process minimizes the chances of contamination and ensures that the deposited material forms a high-purity film. The controlled heating and subsequent condensation allow for precise control over the film's thickness and uniformity, making evaporation coating a versatile and effective method for various applications.
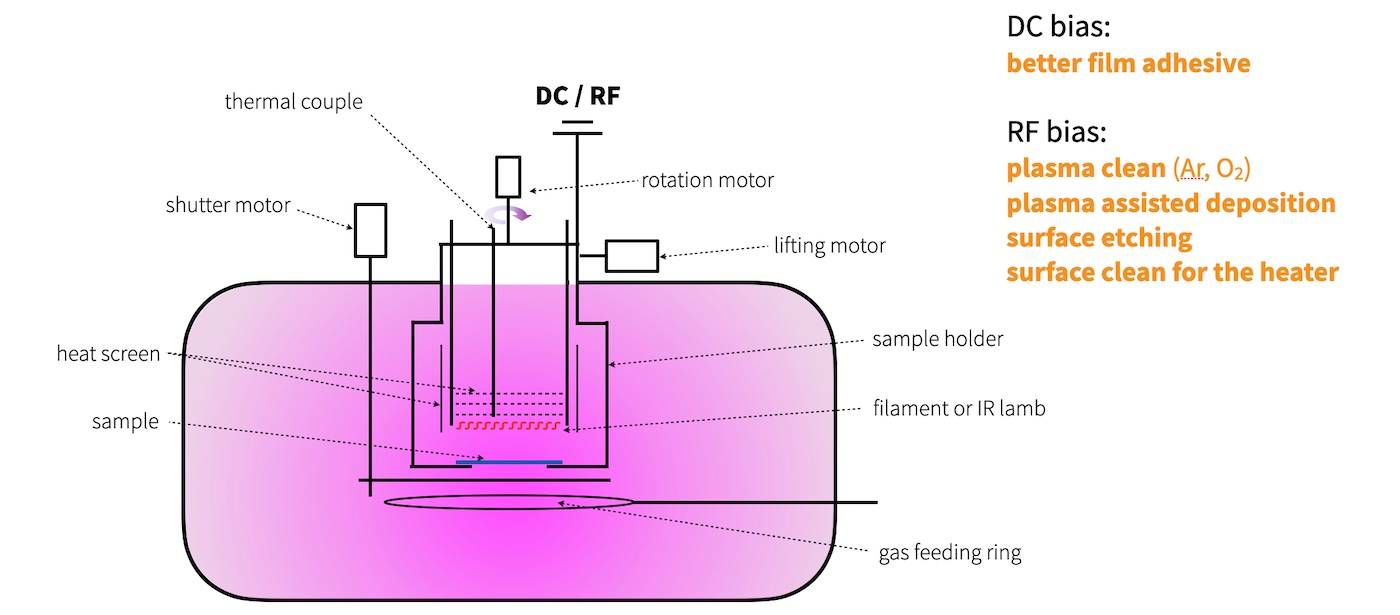
Types of Evaporation Sources
Evaporation sources are crucial components in the process of evaporation plating, each designed to heat and vaporize materials efficiently. The primary types of evaporation sources include:
-
Resistance Heating Source: This method involves passing a large current through a resistive wire or foil containing the material to be deposited. The heating element, often referred to as an "evaporation source," can be made from materials like tungsten wire, which can be formed into various shapes such as filaments, baskets, heaters, or looped point sources. This method is particularly effective for materials with high melting points and low vapor pressures.
-
High-Frequency Induction Heating Source: Utilizing high-frequency electromagnetic fields, this source heats the evaporation material indirectly. The material is placed in a crucible, which is then heated by the induction currents. This method is advantageous for materials that require precise temperature control and are sensitive to direct heating methods.
-
Electron Beam Heating Source: In this advanced method, the evaporation source is heated by an electron beam with energies up to 15 keV. The high-energy electron beam allows for the precise control of heating, making it suitable for materials that require very high temperatures to vaporize. This method is particularly useful for refractory materials and those with high melting points.
-
Flash Evaporation: An alternative method, flash evaporation involves continuously feeding fine wire or powder of the source material onto a hot ceramic or metallic bar, causing it to evaporate on contact. This method is efficient for materials that can be easily powdered or wire-formed, providing a continuous and rapid evaporation process.
-
Filament Evaporation: This traditional method places the evaporation metal source on filaments made from materials such as tungsten, molybdenum, quartz, or graphite. The metal is heated to its melting point by passing a large current through the resistive wire or foil, creating a pool of melted metal that evaporates into a cloud above the source.
Each of these methods offers unique advantages and is selected based on the specific requirements of the material to be evaporated and the desired characteristics of the deposited film.
Characteristics
Evaporation plating offers unparalleled versatility, capable of depositing metals, semiconductors, insulators, and even alloys and compounds onto a wide array of substrates, including metals, semiconductors, insulators, plastics, papers, and fabrics. This broad applicability sets it apart from other deposition methods. The process can yield films with varying microstructures and crystalline morphologies—ranging from monocrystalline to polycrystalline or amorphous—by adjusting parameters such as deposition rates, substrate temperatures, and the incidence angle of vapor molecules.
Moreover, evaporation plating ensures exceptionally high film purity and facilitates real-time monitoring and control of film thickness and composition. The precision of thickness control can reach the level of a single molecular layer, making it a highly precise technique for various applications. This level of control and adaptability underscores its extensive use in industries requiring precise and diverse film properties.
Sputtering Plating
Definition and Principle
In the process of sputtering plating, high-energy particles are directed towards the surface of a solid material, causing the surface particles to gain sufficient energy to escape and subsequently deposit onto a substrate. The material intended for deposition is typically shaped into a plate known as the target, which is securely mounted on the cathode. The substrate, positioned on the anode, faces the target at a distance of just a few centimeters.
Prior to the initiation of the sputtering process, the system is evacuated to achieve a high vacuum, typically filled with a gas pressure of 10 to 1 Pa, most commonly argon. A voltage of several thousand volts is then applied between the cathode and anode, creating a glow discharge that ionizes the gas. The positive ions generated by this discharge are accelerated by the electric field towards the cathode, where they collide with the target's surface atoms. These collisions result in the ejection of target atoms, known as sputtered atoms, which possess energies ranging from 1 to dozens of electron volts.
The sputtered atoms, upon escaping the target, travel through the vacuum and eventually condense onto the substrate's surface, forming a thin film. This method of deposition is particularly effective due to the high degree of control over the energy and direction of the sputtered atoms, which ensures uniform and robust film formation.
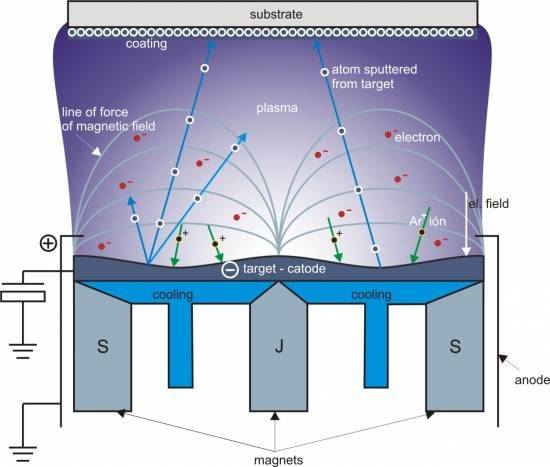
Classification
Sputtering plating techniques are categorized into several distinct methods, each with its own unique operational principles and applications. The primary classifications include:
-
Reaction Sputtering Method: This method involves introducing reactive gases into the sputtering chamber to form compound films. For instance, introducing oxygen during the sputtering of titanium can result in the formation of titanium oxide films. This technique is particularly useful for creating functional films with specific chemical properties.
-
High-Frequency Sputtering Method: Utilizing high-frequency electrical fields, this method allows for the sputtering of materials that are not conductive at low frequencies. It is particularly effective for insulating materials and complex alloys, enabling a broader range of materials to be coated.
-
Others: Beyond the aforementioned methods, there are additional specialized techniques such as Magnetron Sputtering and Reactive Sputtering. Magnetron sputtering enhances the efficiency of the sputtering process by using a magnetic field to confine electrons, thereby increasing the ionization of the sputtering gas. Reactive sputtering, on the other hand, involves the use of reactive gases to create compound films, similar to the reaction sputtering method but with more precise control over the chemical composition of the deposited film.
Each of these methods offers distinct advantages and is suited for different types of applications, contributing to the versatility and effectiveness of sputtering plating as a whole.
Characteristics
Sputtering coating offers several distinct advantages over other deposition methods. One of its most notable features is its ability to sputter a wide range of materials, including refractory substances like tungsten (W), tantalum (Ta), carbon (C), molybdenum (Mo), tungsten carbide (WC), and titanium carbide (TiC). This versatility is due to the fact that sputtering is not constrained by the melting point of the film material, making it highly suitable for materials that are difficult to process through traditional evaporation methods.
The process yields a plating layer that exhibits strong adhesion to the substrate, ensuring durability and longevity. This adhesion is complemented by the density and uniformity of the coating, which are critical for maintaining the integrity and performance of the final product. Unlike processes where gravity plays a significant role, sputtering allows for the free arrangement of the target and substrate, enabling precise control over the deposition process.
During the initial stages of film formation, sputtering achieves a high nucleation density, which is essential for producing extremely thin, continuous films—even those below 10 nanometers. This capability is particularly valuable in applications requiring delicate and precise coatings. Additionally, the target material in sputtering has a long service life, facilitating long-term, continuous production that can be easily automated.
The flexibility in shaping the target further enhances the process's efficiency. Targets can be engineered into various forms, allowing for specialized designs that optimize control and production rates. Sputtering typically employs a high-voltage electric field to generate plasma, which can be used to coat materials with a broad spectrum of high-melting-point metals, alloys, and metal oxides, including chromium, molybdenum, tungsten, titanium, silver, and gold.
Despite its numerous advantages, sputtering does come with a higher processing cost compared to some other methods. This cost is often justified by the superior quality and versatility of the coatings produced, making it a preferred choice in industries where performance and reliability are paramount.
Ion Plating
Definition and Principle
In ion plating, the molecules of an evaporated substance undergo ionization through electron collisions, ultimately depositing as ions onto a solid surface. This process is known as ion plating. The setup involves connecting the evaporation source to the anode and the workpiece to the cathode. When a high-voltage direct current (typically between three to five kilovolts) is applied, a glow discharge is generated between the evaporation source and the workpiece.
Under vacuum conditions, the chamber is filled with inert argon gas. The electric field from the discharge ionizes a portion of the argon, creating a plasma dark zone around the cathode workpiece. Positively charged argon ions are attracted to the negatively charged cathode, bombarding the surface of the workpiece with significant force. This bombardment effectively cleans the surface by dislodging particles and contaminants, preparing it for the deposition process.
Subsequently, the evaporation source is connected to an AC power supply, causing the evaporation material particles to melt and evaporate. These particles enter the glow discharge area where they are ionized. The positively charged evaporation material ions, along with the argon ions, are drawn towards the cathode and deposited onto the workpiece. When the number of ions deposited exceeds those lost through sputtering, a layer of solid adhesion forms on the workpiece surface, gradually building up the plating layer.
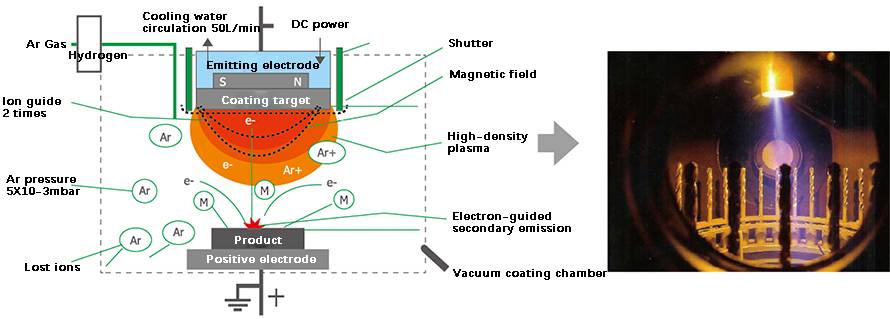
Classification
Ion plating techniques are diverse, each designed to address specific requirements and challenges in the deposition process. The primary classifications include:
-
Magnetron Sputtering Ion Plating: This method utilizes a magnetic field to enhance the sputtering process, increasing the efficiency of ion generation and deposition. It is particularly effective for high-throughput applications and can handle a variety of target materials.
-
Reaction Ion Plating: In this technique, reactive gases are introduced during the ion plating process to form compound films, such as oxides, nitrides, or carbides. This allows for the creation of functional coatings with specific properties, such as enhanced hardness or corrosion resistance.
-
Hollow Cathode Discharge Ion Plating: This method employs a hollow cathode to generate a high-density plasma, which facilitates a more uniform and controlled deposition. It is ideal for applications requiring precise control over film thickness and composition.
-
Multi-Arc Ion Plating: This technique involves multiple arc sources to create a more intense plasma, enabling the deposition of thicker and more adherent coatings. It is commonly used for applications requiring high durability and wear resistance.
Each of these methods offers unique advantages and is suited to different types of applications, making ion plating a versatile and powerful tool in materials science and engineering.
Characteristics
Ion plating exhibits several distinctive characteristics that set it apart from other plating methods. One of the most notable features is its excellent adhesion performance. During tensile tests, ion-plated specimens demonstrate remarkable resilience. Even when stretched to the brink of fracture, the plating layer remains firmly attached to the base metal, exhibiting plastic extension without any signs of peeling or flaking.
Another significant advantage of ion plating is its superior coverage capability. This method is particularly adept at plating parts with intricate geometries, such as those with holes, grooves, and narrow slits. Traditional plating techniques often struggle with such complex shapes, making ion plating a preferred choice for these applications.
The quality of the plating produced by ion plating is also noteworthy. The resulting coatings are characterized by their dense, pinhole-free structure, devoid of bubbles, and with uniform thickness. This high-quality finish ensures durability and longevity, making ion-plated components highly reliable.
Moreover, the simplified cleaning process associated with ion plating further enhances its appeal. Unlike other methods that require extensive post-plating cleaning, ion plating reduces the need for such elaborate procedures, streamlining the overall process and reducing operational costs.
| Characteristic | Description |
|---|---|
| Adhesion Performance | Excellent adhesion; no peeling or flaking even under extreme tensile conditions. |
| Coverage Capability | Ideal for plating complex shapes like holes, grooves, and narrow slits. |
| Plating Quality | Dense, pinhole-free, bubble-free, and uniform thickness. |
| Cleaning Process | Simplified, reducing operational costs and streamlining the process. |
These characteristics collectively make ion plating a versatile and efficient method for a wide range of applications, particularly in industries where high-quality and durable coatings are essential.
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Electron Beam Evaporation Coating Gold Plating Tungsten Molybdenum Crucible for Evaporation
- Electron Beam Evaporation Coating Tungsten Crucible and Molybdenum Crucible for High Temperature Applications
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use