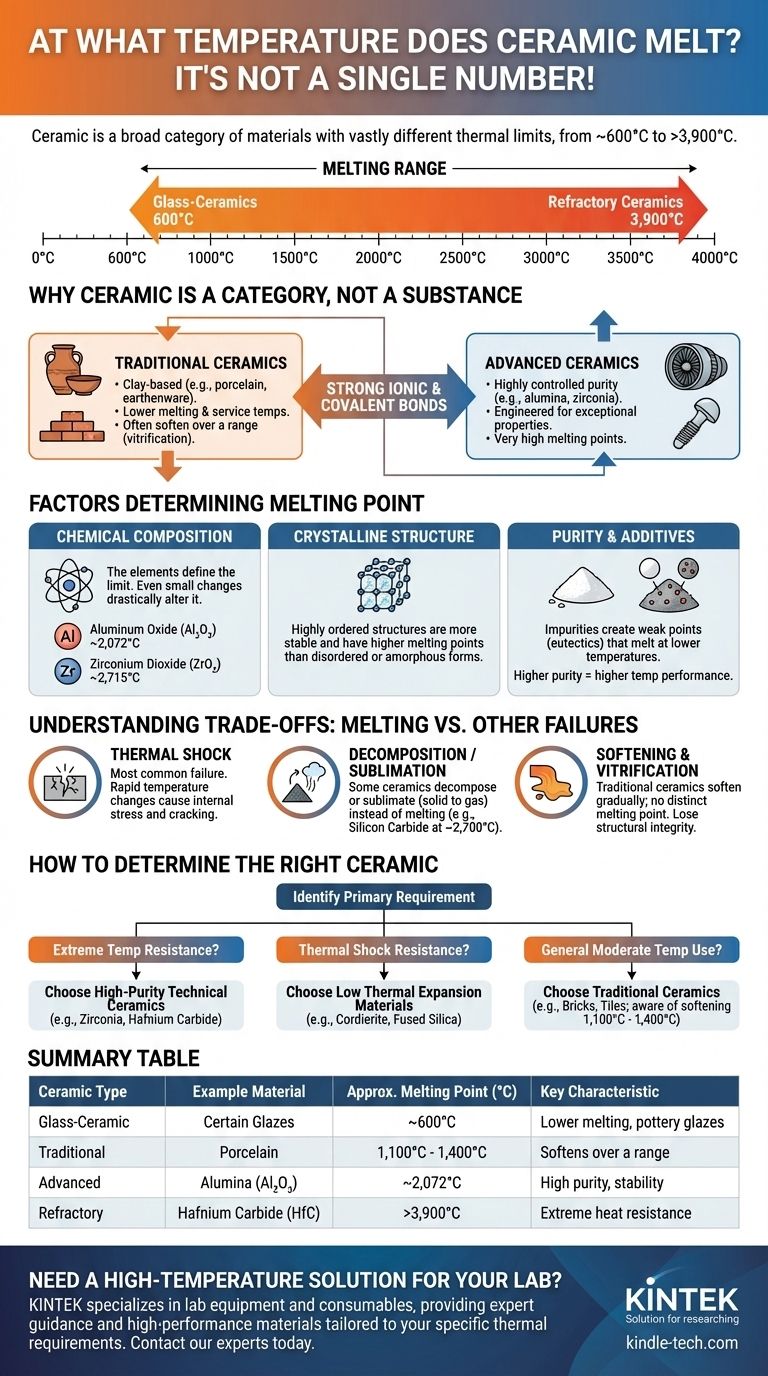
Unlike a simple element like iron, there is no single melting point for ceramic. The term "ceramic" covers a vast category of materials whose melting temperatures vary dramatically based on their specific chemical composition and structure. This range spans from as low as 600°C (1,112°F) for certain glass-ceramics to well over 3,900°C (7,050°F) for advanced, refractory ceramics like hafnium carbide.
The critical question is not "at what temperature does ceramic melt," but rather "what is the specific composition and crystalline structure of the ceramic in question?" These two factors fundamentally dictate its behavior at high temperatures.

Why "Ceramic" is a Category, Not a Substance
To understand thermal limits, you must first understand what a ceramic is and is not. This distinction is the key to selecting the right material.
The Definition of a Ceramic
A ceramic is a solid material comprising an inorganic compound of metal, non-metal, or metalloid atoms held together by strong ionic and covalent bonds. They are typically formed by the action of heat.
This broad definition includes everything from common pottery and bricks to advanced components in jet engines and medical implants.
Traditional vs. Advanced Ceramics
The world of ceramics is best understood by splitting it into two groups.
Traditional ceramics are clay-based products like brick, porcelain, and earthenware. Their properties are variable, and they generally have much lower melting and service temperatures.
Advanced ceramics (also called technical or engineering ceramics) are materials with highly controlled purity and composition, engineered for exceptional properties. This group includes materials like alumina, zirconia, and silicon carbide, which are known for extreme heat resistance.
The Power of Chemical Bonds
The defining characteristic of most ceramics is the strength of their atomic bonds. Ionic and covalent bonds are significantly stronger than the metallic bonds found in metals.
Breaking these powerful bonds requires an immense amount of thermal energy, which is the fundamental reason ceramics have such high melting points compared to most metals.
Factors That Determine a Ceramic's Melting Point
The specific temperature at which a ceramic melts or fails is not a random number. It is a direct result of its internal makeup.
Chemical Composition
The elements that make up the ceramic are the single most important factor. Even a small change in composition can drastically alter the melting point.
For example, Aluminum Oxide (Al₂O₃), a very common technical ceramic, melts at approximately 2,072°C (3,762°F).
In contrast, Zirconium Dioxide (ZrO₂), another advanced ceramic, melts at a much higher 2,715°C (4,919°F).
Crystalline Structure
The way atoms are arranged in a rigid, repeating lattice also impacts stability. A tightly packed and highly ordered crystalline structure is more difficult to break apart than a less-ordered one.
This is why a fully dense, single-crystal ceramic will typically have a higher and sharper melting point than its powdered or less-ordered polycrystalline form.
Purity and Additives
Impurities or intentionally added binding agents can create weak points within the ceramic's structure. These points often form "eutectics," which are mixtures that melt at a lower temperature than the pure components surrounding them.
This is why a 99.9% pure alumina has superior high-temperature performance compared to a 94% pure grade, which contains other glassy phases that soften and melt much sooner.
Understanding the Trade-offs: Melting vs. Other Failures
Reaching the melting point is not the only way a ceramic component can fail under heat. In many real-world applications, other failure modes are far more common and occur at much lower temperatures.
Thermal Shock
This is arguably the most common cause of ceramic failure. Thermal shock occurs when a rapid change in temperature creates internal stresses, causing the material to crack.
A ceramic can have a melting point of 3,000°C but shatter at 400°C if heated or cooled too quickly. Its inherent brittleness makes it vulnerable.
Decomposition or Sublimation
Some ceramics do not melt cleanly at atmospheric pressure. Instead, they may decompose into their constituent elements or sublimate (transform directly from a solid to a gas).
Silicon Carbide (SiC) is a prime example. It begins to decompose around 2,700°C (4,892°F) rather than turning into a stable liquid.
Softening and Vitrification
Traditional, clay-based ceramics rarely have a distinct melting point. Instead, they soften over a wide temperature range as their glassy components begin to flow.
This process, known as vitrification, is essential for firing pottery, but it means there is no single temperature where the material is "melted." The material simply loses its structural integrity gradually.
How to Determine the Right Ceramic for Your Application
To select the correct material, you must move from the general category to the specific demands of your project. Always consult the manufacturer's technical data sheet for the specific grade of material you are considering.
- If your primary focus is extreme temperature resistance (e.g., furnace linings, crucibles): Look for high-purity technical ceramics like Zirconia (ZrO₂), Hafnium Carbide (HfC), or high-purity Alumina (Al₂O₃), as these offer the highest melting points.
- If your primary focus is resistance to thermal shock (e.g., heat exchangers, engine parts): Consider materials engineered for low thermal expansion, such as Cordierite or Fused Silica, as melting point alone is a poor indicator of performance in these scenarios.
- If your primary focus is general use at moderate temperatures (e.g., bricks, tiles): Traditional ceramics are suitable, but be aware they soften and can fail at much lower temperatures (often 1,100°C - 1,400°C) than technical ceramics.
By identifying the specific type of ceramic and its true failure modes, you can engineer a reliable and effective solution.
Summary Table:
| Ceramic Type | Example Material | Approximate Melting Point (°C) | Key Characteristic |
|---|---|---|---|
| Glass-Ceramic | Certain Glazes | ~600°C | Lower melting, used in pottery |
| Traditional | Porcelain | 1,100°C - 1,400°C | Softens over a range |
| Advanced | Alumina (Al₂O₃) | ~2,072°C | High purity, excellent stability |
| Refractory | Hafnium Carbide (HfC) | >3,900°C | Extreme heat resistance |
Need a High-Temperature Solution for Your Lab?
Selecting the right ceramic is critical for your application's success. At KINTEK, we specialize in lab equipment and consumables, providing expert guidance and high-performance materials tailored to your specific thermal requirements. Whether you need crucibles, furnace linings, or custom ceramic components, we have the expertise to ensure reliability and efficiency.
Let us help you engineer a reliable solution. Contact our experts today to discuss your project and discover how our advanced ceramics can enhance your laboratory's performance.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is sintered ceramic? A Durable, Non-Porous Material for Modern Surfaces
- Why use Aluminum Nitride (AlN) foil for sintering? Essential Protection for High-Purity Material Synthesis
- What role does a carbon template play in nanocasting magnesium oxide? Master Porous Material Precision
- Is ceramic breakable or unbreakable? Understanding the Strength and Brittleness of Ceramics
- How do alumina ceramic tubes address technical challenges in electrochemical devices? Ensure Peak Signal Integrity.
- Is ceramic temperature sensitive? Master Thermal Shock for Peak Performance
- What is meant by ceramic powder? The Engineered Blueprint for Advanced Ceramics
- Do ceramics have corrosion resistance? Leverage Their Inert Nature for Demanding Applications



















