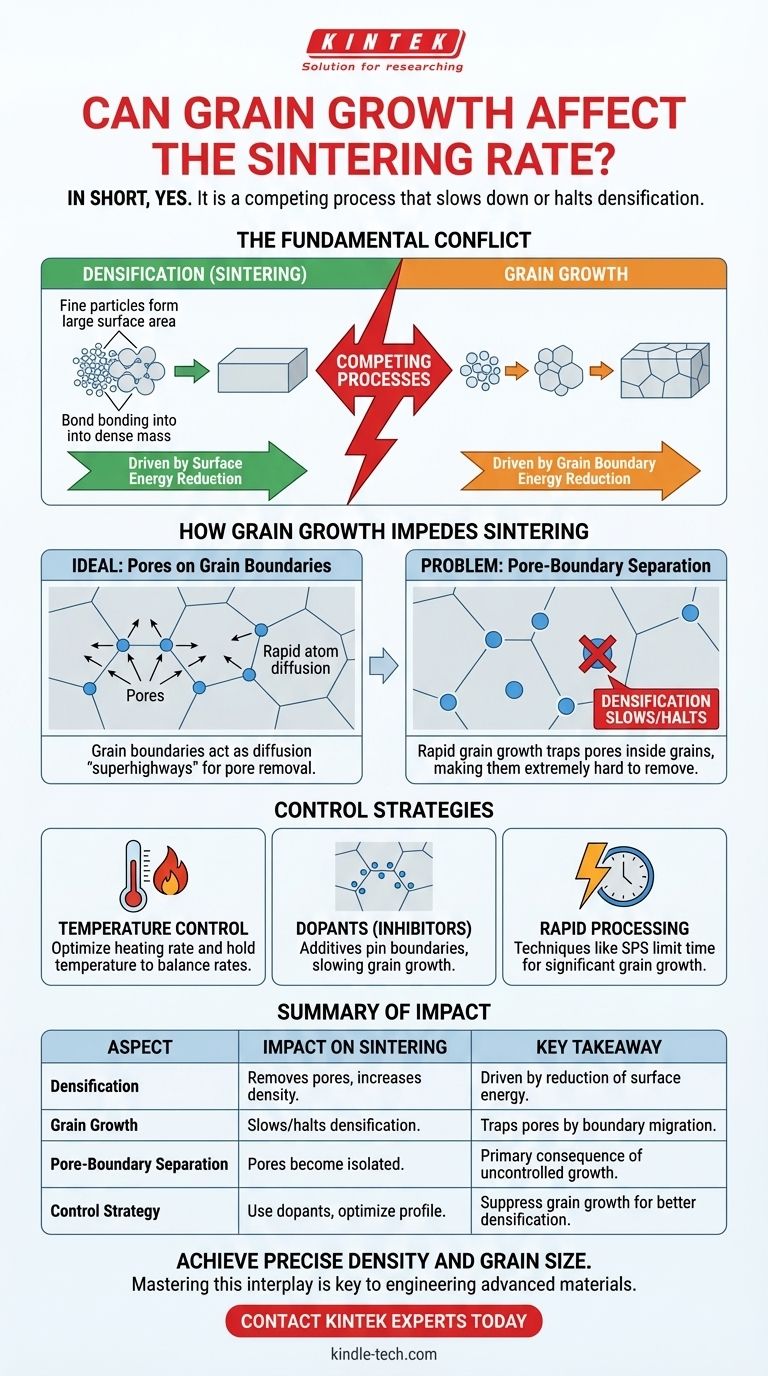
In short, yes. Grain growth critically affects the sintering rate, and in most cases, it is a competing process that slows down or even halts densification. The relationship between these two phenomena is one of the most fundamental challenges in powder processing and the manufacturing of advanced ceramics and metals.
The core issue is that both sintering (densification) and grain growth are driven by the reduction of energy in the material system at high temperatures. However, grain growth can eliminate the very diffusion pathways—the grain boundaries—that are essential for efficiently removing pores and achieving high density.

The Fundamental Conflict: Densification vs. Grain Growth
To control the outcome of a sintering process, it is essential to understand the two mechanisms at play. They occur simultaneously but are driven by different, though related, energy reductions.
The Driving Force for Sintering
Sintering is the process by which a collection of particles bonds together at high temperatures to form a dense, solid mass. This process is driven by the desire to reduce the system's total surface energy.
Fine powders have an enormous amount of surface area. By forming necks between particles and eventually eliminating the pores between them, the material drastically reduces this high surface energy, which is a thermodynamically favorable process. This removal of pores is what we call densification.
The Driving Force for Grain Growth
A sintered material is composed of many individual crystals, or grains. The interface between any two grains is a grain boundary, which is a region of higher energy compared to the perfect crystal lattice inside the grain.
The system can reduce its total energy by minimizing the total area of these grain boundaries. This is achieved as larger grains grow at the expense of smaller grains, a process known as grain growth or coarsening.
How Grain Growth Directly Impedes Sintering
The problem arises because the primary mechanism for densification relies heavily on the presence and location of grain boundaries.
The Critical Role of Grain Boundaries
Grain boundaries act as "superhighways" for the diffusion of atoms. For a pore to be eliminated, atoms must move from the grain boundary surface to fill the empty space of the pore. This process, grain boundary diffusion, is much faster than diffusion through the crystal lattice itself.
For efficient densification, pores must remain attached to the grain boundaries.
The Separation of Pores from Boundaries
During grain growth, grain boundaries migrate. If a boundary moves too quickly, it can break away from a pore, leaving the pore stranded inside a large grain. This event is called pore-boundary separation.
The Consequence of Trapped Pores
Once a pore is isolated within a grain, it is extremely difficult to remove. The only way to fill it is through the much slower process of lattice diffusion.
At this point, the rate of densification drops dramatically. This is why uncontrolled grain growth is the primary obstacle to achieving full theoretical density in many materials.
Understanding the Trade-offs and Control Strategies
Managing the competition between densification and grain growth is the central task of optimizing any sintering process.
The Impact of Temperature
Higher temperatures accelerate both atomic diffusion for densification and grain boundary migration for grain growth. However, they often affect the two rates differently.
A common strategy involves carefully designing a temperature profile (e.g., heating rate, hold temperature, and duration) that maximizes the densification rate relative to the grain growth rate.
The Power of Dopants (Grain Growth Inhibitors)
One of the most effective methods for controlling grain growth is the use of dopants. These are small amounts of a secondary material added to the primary powder.
Dopant atoms tend to segregate at the grain boundaries. This creates a "solute drag" effect, which effectively pins the boundaries and makes it more difficult for them to migrate. By slowing down grain growth, dopants allow the densification process to continue to a much later stage, enabling the removal of more pores and achieving higher final densities.
Making the Right Choice for Your Goal
The ideal sintering strategy depends entirely on the desired properties of the final component. Your approach should be tailored to manage the densification-grain growth balance accordingly.
- If your primary focus is achieving maximum density: Your main goal is to suppress premature grain growth. Consider using finer starting powders, exploring lower sintering temperatures for longer times, or introducing specific grain growth inhibiting dopants.
- If your primary focus is controlling the final grain size (for mechanical or optical properties): You must carefully manage the entire time-temperature profile. Advanced techniques like two-step sintering or hot pressing can provide more precise control over the final microstructure.
- If your primary focus is rapid processing: You may need to accept some compromise in density or grain size. High-speed methods like Spark Plasma Sintering (SPS) can densify materials in minutes, often limiting the time available for significant grain growth to occur.
Ultimately, mastering the interplay between grain growth and sintering is the key to engineering materials with precisely tailored microstructures and properties.
Summary Table:
| Aspect | Impact on Sintering | Key Takeaway |
|---|---|---|
| Densification | Removes pores, increases density. | Driven by reduction of surface energy. |
| Grain Growth | Slows/halts densification by trapping pores. | Driven by reduction of grain boundary energy. |
| Pore-Boundary Separation | Pores become isolated, extremely hard to remove. | The primary consequence of uncontrolled grain growth. |
| Control Strategy | Use of dopants, optimized temperature profiles. | Suppress grain growth to allow densification to continue. |
Achieve the precise density and grain size your application demands. The competition between sintering and grain growth is a fundamental challenge in powder processing. KINTEK specializes in providing the lab equipment, consumables, and expert support to help you master your sintering process. Whether you are developing advanced ceramics or high-performance metal parts, we can help you optimize your parameters for superior results. Contact our experts today to discuss how we can support your laboratory's specific needs.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How does a high-temperature annealing furnace regulate ODS steel performance? Optimize Microstructure for Superior Strength
- What is the melting point of an arc furnace? Understanding Its Extreme Heat for Metal Melting
- Can stainless steel be brazed? Yes, with the right techniques to overcome chromium oxide
- What are the benefits of tempering? Achieve the Perfect Balance of Hardness and Toughness
- What function does a high-temperature annealing furnace perform? Enhance TiO2 Thin Film Properties & Crystal Structure
- What is the purpose of using a high-temperature vacuum degassing furnace? Ensure High-Density ODS FeCrAl Alloy Quality
- Why do you vacuum braze? Achieve Superior Joint Integrity for Mission-Critical Components
- Is vacuum hardening better than normal hardening? A Guide to Precision vs. Cost-Effectiveness



















