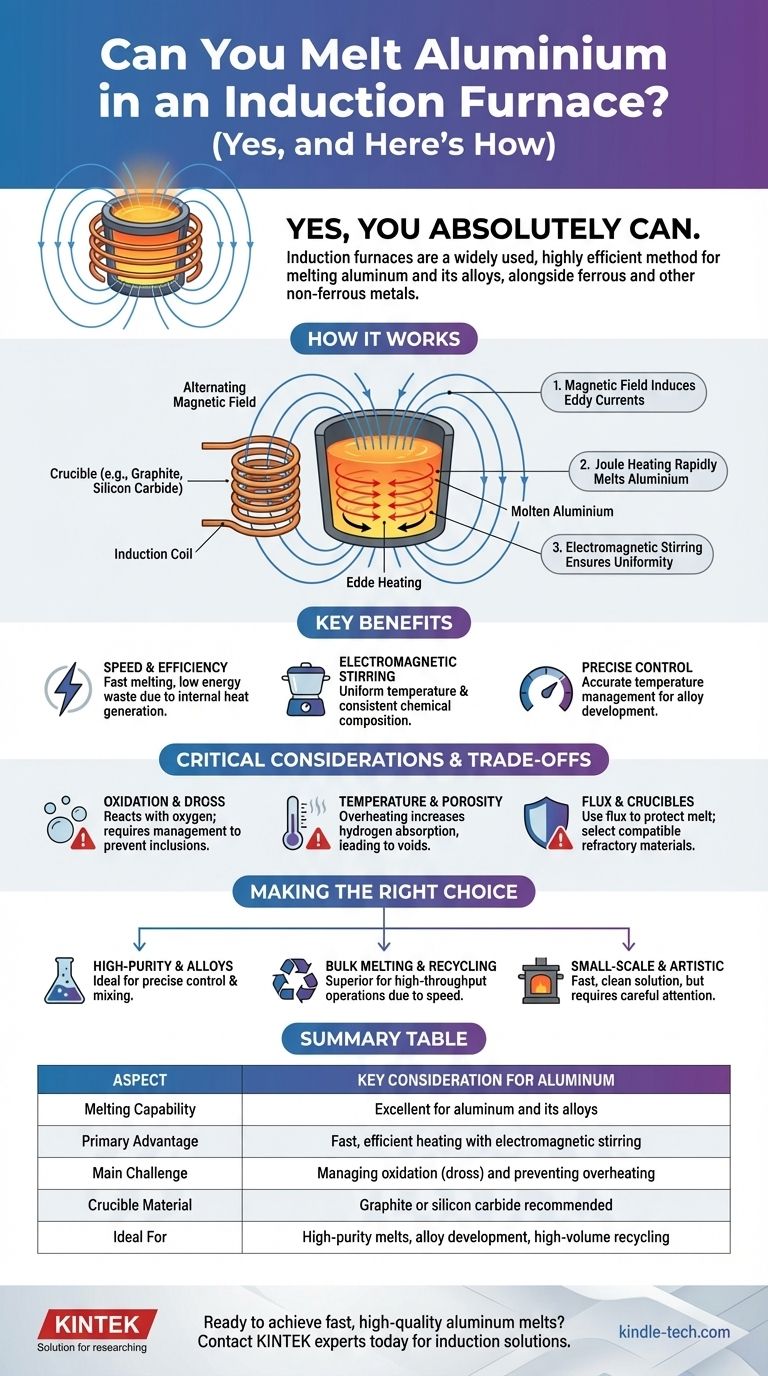
Yes, you absolutely can. An induction furnace is not only capable of melting aluminum and its alloys, but it is a widely used and highly efficient method for doing so. The technology works for a vast range of both ferrous metals, like iron and steel, and non-ferrous metals, including copper, brass, and aluminum.
The core takeaway is that while induction furnaces are excellent for melting aluminum due to their speed and efficiency, success depends on managing the unique properties of aluminum. Controlling oxidation and temperature is more critical than achieving maximum power.

How Induction Furnaces Work for Aluminum
An induction furnace doesn't use an external flame or heating element. Instead, it uses the principles of electromagnetism to heat the metal directly, resulting in a fast, clean, and controllable melting process.
The Principle of Induction Heating
The furnace generates a powerful, alternating magnetic field around a crucible containing the aluminum. This magnetic field induces strong electrical currents, known as eddy currents, directly within the metal. The aluminum's natural electrical resistance causes these currents to generate intense heat, a phenomenon called Joule heating, quickly raising the metal to its melting point.
The Advantage of Electromagnetic Stirring
A significant benefit of this process is the natural stirring action created by the magnetic forces. This electromagnetic stirring ensures the molten aluminum is constantly mixed, leading to a uniform temperature and consistent chemical composition throughout the melt. This is especially valuable when creating specific aluminum alloys by adding other elements.
Speed and Efficiency
Induction furnaces are exceptionally fast. Depending on the size and power of the unit, a batch of aluminum can be melted in minutes. Because the heat is generated inside the metal itself, very little energy is wasted, making it a highly efficient process compared to traditional fuel-fired furnaces.
Key Considerations and Trade-offs
While effective, melting aluminum with induction requires careful management. The properties that make aluminum a valuable material also present unique challenges during the melting process.
Managing Oxidation and Dross
Aluminum reacts very readily with oxygen in the air, forming a layer of aluminum oxide (dross) on the surface of the melt. The vigorous stirring action of an induction furnace can sometimes fold this dross back into the molten metal, creating inclusions that compromise the quality of the final casting.
The Critical Role of Temperature Control
Induction furnaces can achieve extremely high temperatures, but for aluminum, this power must be carefully controlled. Overheating the melt significantly increases oxidation and can cause it to absorb hydrogen gas from the atmosphere. This dissolved gas leads to porosity, a critical defect that creates tiny voids in the solidified metal, severely weakening it.
Using Flux to Protect the Melt
To combat oxidation, a layer of flux is often added to the top of the molten aluminum. This material creates a protective barrier against the atmosphere and helps agglomerate dross, making it easier to skim off before pouring. This addresses the challenge of managing impurities and oxidants.
Choosing the Right Crucible
The crucible, which holds the metal, must be made from a refractory material that can withstand high temperatures and does not react with molten aluminum. Graphite and silicon carbide are common and effective choices for aluminum melting applications.
Making the Right Choice for Your Application
An induction furnace is a powerful tool for melting aluminum, but its application should align with your specific goals.
- If your primary focus is high-purity melts or alloy development: The precise temperature control and excellent mixing action of an induction furnace are ideal. A vacuum induction furnace offers the ultimate protection from atmospheric contamination.
- If your primary focus is bulk melting or recycling scrap: The sheer speed and energy efficiency of induction technology make it a superior choice for high-throughput operations.
- If your primary focus is small-scale or artistic casting: Smaller induction furnaces provide a fast, clean, and relatively compact solution, but careful attention to managing dross and temperature remains essential for quality results.
By understanding and controlling the process, induction melting becomes one of the most effective methods for producing high-quality molten aluminum.
Summary Table:
| Aspect | Key Consideration for Aluminum |
|---|---|
| Melting Capability | Excellent for aluminum and its alloys |
| Primary Advantage | Fast, efficient heating with electromagnetic stirring |
| Main Challenge | Managing oxidation (dross) and preventing overheating |
| Crucible Material | Graphite or silicon carbide recommended |
| Ideal For | High-purity melts, alloy development, and high-volume recycling |
Ready to achieve fast, high-quality aluminum melts?
KINTEK specializes in precision lab equipment, including induction furnaces perfect for your aluminum melting and alloy development needs. Our solutions offer the precise temperature control and efficiency required to manage oxidation and produce superior results.
Contact our experts today to find the ideal induction melting system for your laboratory!
Visual Guide
Related Products
- Lab-Scale Vacuum Induction Melting Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Induction Melting Spinning System Arc Melting Furnace
People Also Ask
- What principle is used to generate heat in a vacuum induction melting furnace? Achieve Clean, Efficient Metal Melting
- What types of metals are typically processed in a vacuum induction melting furnace? High-Purity Alloys for Critical Applications
- How does induction work in a vacuum? Achieve Ultra-Pure Metal Melting with VIM
- What is the vacuum induction method? Master High-Purity Metal Melting for Advanced Alloys
- What is VIM in metallurgy? A Guide to Vacuum Induction Melting for High-Performance Alloys



















