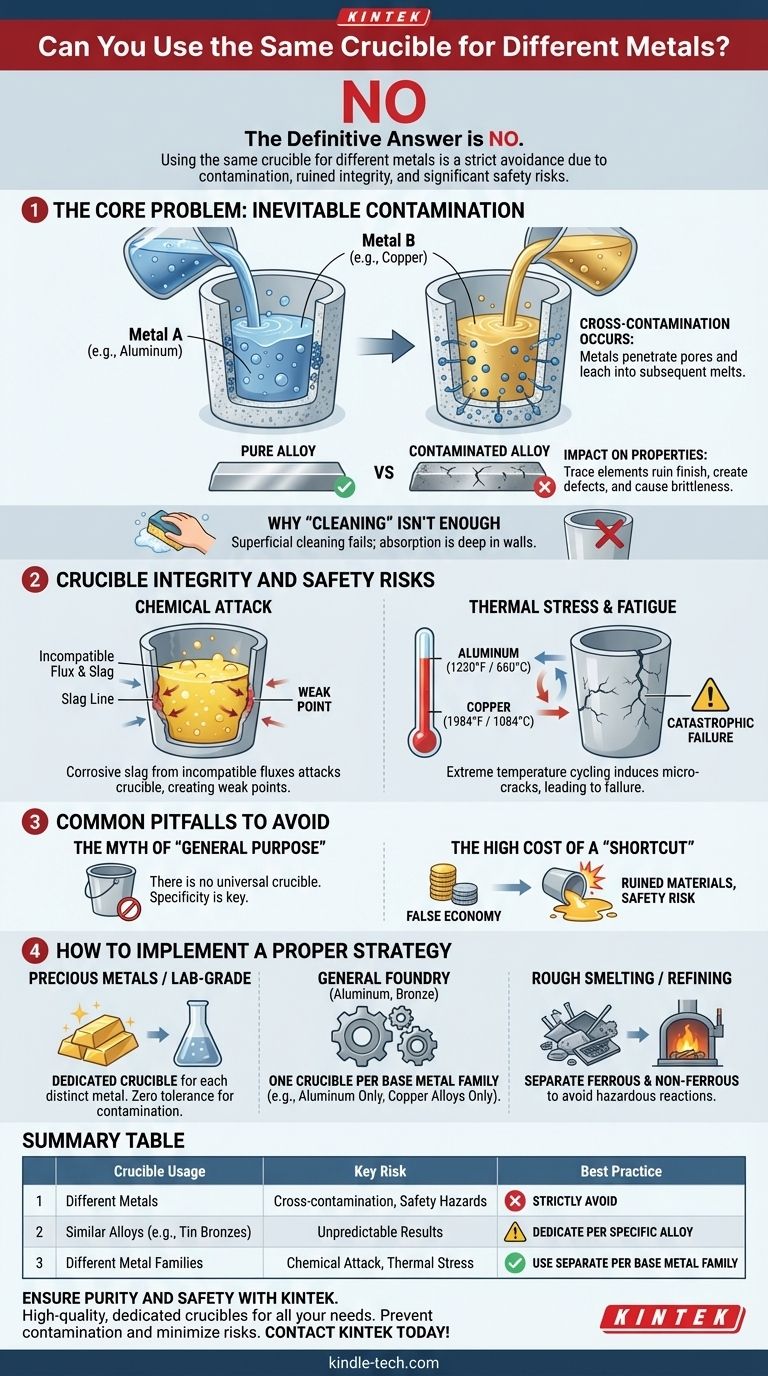
The definitive answer is no. Using the same crucible for different metals is a practice that should be strictly avoided in almost all situations. Doing so introduces contamination that can ruin the integrity of your metals and accelerates the degradation of the crucible itself, posing a significant safety risk.
The core principle is simple: a crucible absorbs trace amounts of every metal melted within it. This unavoidable contamination compromises the quality of subsequent melts and weakens the crucible over time, making dedicated crucibles—one for each type of metal—a fundamental requirement for safe and reliable work.

The Core Problem: Inevitable Contamination
The primary reason to dedicate crucibles to a single metal or alloy family is to prevent cross-contamination. This isn't just a matter of surface-level cleaning; it's an issue of material science.
How Cross-Contamination Occurs
Crucibles, whether made of graphite, clay-graphite, or silicon carbide, are porous on a microscopic level. When metal is molten, it penetrates these pores and becomes permanently embedded in the crucible's structure.
No amount of scraping, grinding, or re-firing can completely remove this absorbed material. The next time you heat the crucible with a different metal, these trapped elements will leach out and mix into the new melt.
The Impact on Metal Properties
Even minute amounts of a foreign element can have a dramatic, negative effect on an alloy's intended properties.
For example, a small amount of aluminum contamination in a bronze melt can create casting defects and ruin the metal's finish. Similarly, trace amounts of lead left in a crucible used for gold or silver will make the precious metals brittle and unworkable.
Why "Cleaning" Isn't Enough
A common misconception is that a crucible can be "cleaned out" between melts. While you should always remove dross and visible residue after a pour, this is purely superficial.
The true contamination is absorbed into the crucible walls. Attempting to burn it out is often futile and can cause thermal shock, further damaging the crucible.
Beyond Contamination: Crucible Integrity and Safety
Using one crucible for different metals doesn't just damage your final product; it actively damages the crucible and introduces serious safety hazards.
Chemical Attack from Flux and Slag
Different metals and alloys often require specific types of flux for cleaning and purification. These fluxes react with impurities to form slag, which is highly corrosive.
A flux suitable for aluminum can aggressively attack a crucible at the higher temperatures required for bronze. This chemical wear creates a weak point—often at the slag line—where a catastrophic failure is most likely to occur.
Thermal Stress and Material Fatigue
Subjecting a single crucible to wildly different temperature ranges creates immense thermal stress. The expansion and contraction from cycling between the melting point of aluminum (1220°F / 660°C) and copper (1984°F / 1084°C) will induce micro-cracks.
Over time, these cracks propagate, severely weakening the crucible's structure until it fails completely, often while full of molten metal.
Common Pitfalls to Avoid
Adhering to best practices prevents costly and dangerous mistakes. Understanding the false economies some people chase is key to developing a professional workflow.
The Myth of a "General Purpose" Crucible
There is no such thing as a "universal" or "general purpose" crucible. Any tool designed for high-performance applications requires specificity. A crucible is a precision tool, not a generic bucket.
What About Similar Alloys?
The only potential exception is using a crucible for very similar alloys within the same family, such as different types of tin bronze. However, the best practice is still to dedicate a crucible to each specific, known alloy to guarantee predictable results. Never mix base metals, such as copper alloys and aluminum alloys.
The High Cost of a "Shortcut"
Attempting to save a small amount of money by reusing a crucible is a classic false economy. The cost of a new crucible is insignificant compared to the cost of ruined materials, wasted time, and especially the immense safety risk of a crucible failure and a molten metal spill.
How to Implement a Proper Crucible Strategy
Your approach should be guided by the material you are working with and the quality you need to achieve. A clear system prevents errors.
- If your primary focus is casting precious metals or lab-grade alloys: Use a separate, clearly marked crucible for each distinct metal or alloy. There is zero tolerance for contamination in this context.
- If your primary focus is general foundry work (e.g., aluminum, bronze, brass): Dedicate one crucible for each base metal family. Have an "Aluminum Only" crucible, a "Copper Alloys Only" crucible, and so on.
- If your primary focus is rough smelting or refining scrap: While contamination is less of a concern, it's still wise to separate crucibles for ferrous (iron-based) and non-ferrous metals to avoid unpredictable and potentially hazardous reactions.
Ultimately, treating your crucibles as dedicated tools is the mark of a disciplined and successful metal worker.
Summary Table:
| Crucible Usage | Key Risk | Best Practice |
|---|---|---|
| Same Crucible for Different Metals | Cross-contamination, crucible degradation, safety hazards | Strictly Avoid |
| Similar Alloys (e.g., tin bronzes) | Lower risk, but potential for unpredictable results | Dedicate a crucible per specific alloy |
| Different Metal Families (e.g., aluminum vs. copper) | High risk of chemical attack and thermal stress | Use separate crucibles for each base metal family |
Ensure the purity and safety of your metalwork with the right equipment from KINTEK.
As a leading supplier of lab equipment and consumables, KINTEK provides high-quality crucibles designed for specific metals and alloys. Using dedicated crucibles prevents contamination, extends equipment life, and minimizes safety risks.
Whether you're casting precious metals, conducting foundry work, or refining scrap, we have the right crucible for your needs. Contact us today to discuss your requirements and get expert advice on selecting the best crucible for your application.
Reach out to our specialists now to protect your materials and your workspace.
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
People Also Ask
- What role does an Alumina Crucible play in the high-temperature solid-state synthesis of Na3OBr? Ensure Sample Purity
- What is the function of alumina crucibles in Na3V2(PO4)2F3 synthesis? Ensure Purity in NVPF Production
- Why are high-purity alumina crucibles used for LATP? Preserve Purity and Conductivity in Sintering
- What are the advantages of selecting an alumina crucible for TGA? Ensure High-Precision Thermal Analysis Data
- What are the advantages of high-purity alumina crucibles for molten ZnNaK//Cl salts? Ensure Experimental Purity



















