The correct preparation of rock samples for geochemical analysis is a multi-step process of mechanical size reduction and homogenization. The fundamental procedure involves crushing the initial rock to coarse fragments, splitting it to create a smaller but representative subsample, and finally pulverizing that subsample into a fine, uniform powder ready for instrumental analysis. Each step is designed to ensure the tiny amount of material ultimately analyzed is a true reflection of the original, much larger rock specimen.
The ultimate goal of sample preparation is not merely to crush rocks. It is to systematically transform a large, heterogeneous sample into a small, homogeneous powder from which any portion taken for analysis is chemically and mineralogically identical to the original source.

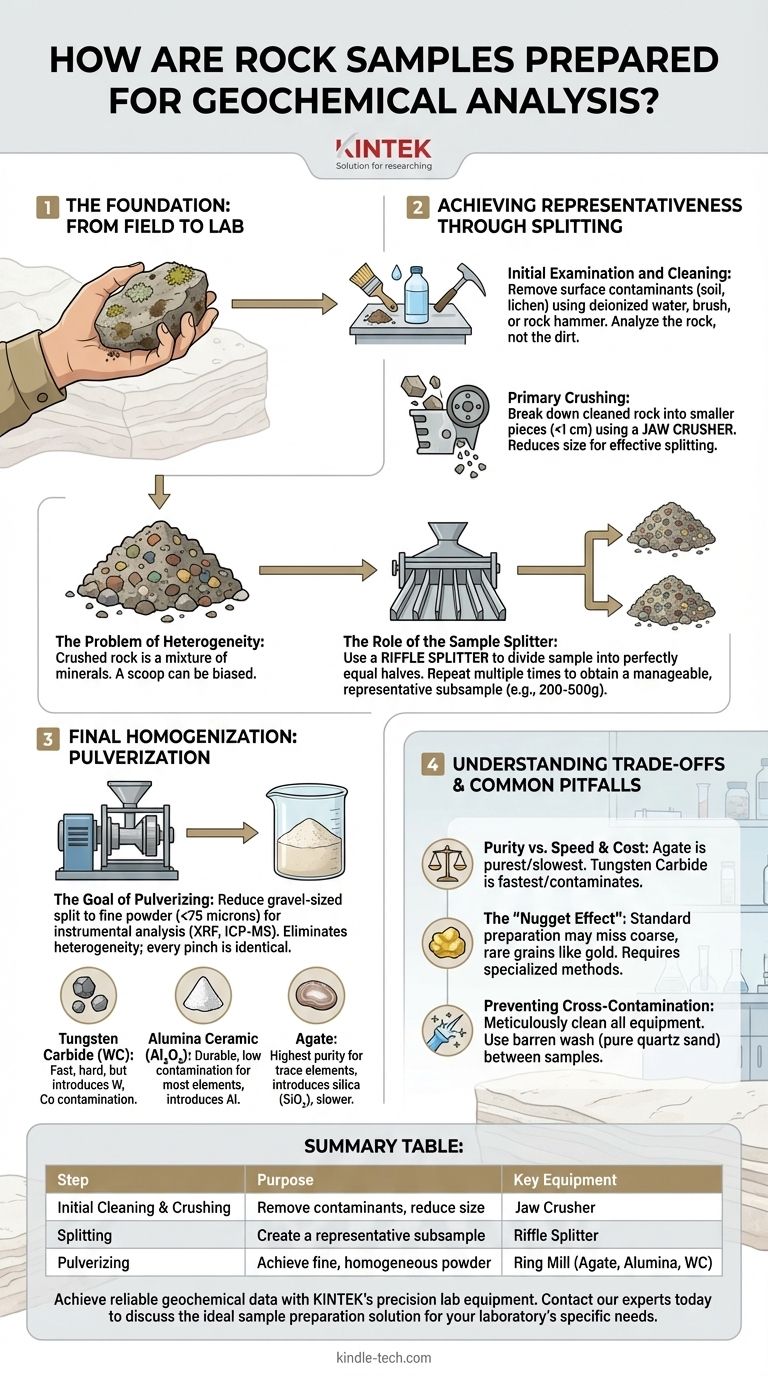
The Foundation: From Field to Lab
The journey from a rock outcrop to an analytical instrument begins with careful handling and primary reduction. This initial stage sets the precedent for the quality of all subsequent data.
Initial Examination and Cleaning
Before any crushing occurs, the sample must be thoroughly inspected. All surface contaminants, such as soil, lichen, moss, or weathering rinds, must be removed.
This is often done by washing the sample with deionized water and a brush, or by physically chipping away the altered outer surfaces with a rock hammer. Failing to do this means you will be analyzing the chemistry of the dirt or lichen on the rock, not the rock itself.
Primary Crushing
The cleaned rock, which may be the size of a fist or larger, is first broken down into smaller, more manageable pieces. This is typically done using a jaw crusher.
A jaw crusher uses two heavy plates—one fixed and one moving—to crush the rock down to a uniform size, usually less than 1 centimeter in diameter. This step doesn't homogenize the sample, but it makes it small enough to be effectively split.
Achieving Representativeness Through Splitting
This is arguably the most critical conceptual step in the entire process. A crushed rock is still a heterogeneous mixture of different minerals, much like a fruitcake is a mixture of cake, nuts, and fruit. Simply scooping a portion is not statistically sound.
The Problem of Heterogeneity
Imagine trying to determine the overall fruit content of a cake by analyzing a single crumb. If that crumb is only cake, your result is 0% fruit. If it's only a raisin, your result is 100% fruit. Neither is correct.
A crushed rock is the same. A scoop might grab a cluster of dark, iron-rich minerals or a cluster of light, silica-rich minerals, biasing the result. The goal of splitting is to overcome this sampling error.
The Role of the Sample Splitter
To create a truly representative subsample, a riffle splitter (or Jones splitter) is used. This device consists of a series of chutes (riffles) that alternate in direction, dividing a sample poured into it into two perfectly equal and identical halves.
The crushed material is passed through the splitter multiple times. One half is discarded and the other is passed through again, repeating until a manageable subsample (e.g., 200-500 grams) is obtained. This ensures the final portion contains the same proportion of minerals as the original bulk sample.
Final Homogenization: Pulverization
The final preparation stage reduces the split, gravel-sized sample into a fine, flour-like powder. This ensures the microscopic portion of material analyzed by an instrument is perfectly homogeneous.
The Goal of Pulverizing
Most modern analytical instruments, like an X-Ray Fluorescence (XRF) spectrometer or an Inductively Coupled Plasma-Mass Spectrometer (ICP-MS), analyze a very small amount of material. The sample is typically pulverized to a very fine powder, often to a size of less than 75 microns (passing through a 200-mesh sieve).
At this fine particle size, the problem of heterogeneity is eliminated. Every pinch of powder is now chemically and mineralogically identical.
Choosing the Right Grinding Material
Pulverizing is done in a high-energy mill, often a ring mill or puck mill. The critical choice here is the material of the grinding vessel itself, as it is the most likely source of contamination.
- Tungsten Carbide (WC): Extremely hard and fast, but will contaminate the sample with tungsten (W) and cobalt (Co), which is used as a binder. It is unsuitable if these are elements of interest.
- Alumina Ceramic (Al₂O₃): A very hard ceramic that offers a good balance of durability and low contamination for most trace elements. It will, however, add aluminum (Al) to the sample.
- Agate: The gold standard for high-purity trace element work. Agate is a form of silica (SiO₂) and introduces negligible contamination for most elements. However, it is less hard and grinding times are significantly longer.
Understanding the Trade-offs and Common Pitfalls
Every choice in sample preparation involves a trade-off. Understanding these is key to producing reliable data.
Purity vs. Speed and Cost
The choice of grinding material is a classic trade-off. Agate provides the highest purity but is the slowest and most expensive option. Tungsten Carbide is fast and efficient, making it ideal for high-throughput commercial labs, but at the cost of known contamination.
The "Nugget Effect"
Standard splitting and pulverizing assumes the elements of interest are finely dispersed. This fails when searching for elements that occur as rare, coarse grains, like gold.
A standard 300-gram split could easily miss a single, tiny gold nugget present in the original 10-kilogram sample. This "nugget effect" requires specialized protocols, such as analyzing a much larger sample portion, to overcome.
Preventing Cross-Contamination
Contamination from the previously prepared sample is a constant threat. All equipment—crushers, splitters, and especially pulverizers—must be meticulously cleaned between every single sample.
The standard cleaning protocol involves a blast of compressed air to remove bulk powder, followed by grinding a "blank" material like pure quartz sand. This barren wash scours the grinding surfaces, removing any lingering residue before the next sample is introduced.
Selecting the Right Preparation Protocol
Your analytical goal dictates the correct preparation method. There is no single "best" way; there is only the best way for your specific question.
- If your primary focus is major element analysis (e.g., Si, Al, Fe using XRF): A robust tungsten carbide mill is often acceptable and efficient, as W and Co are not the target elements.
- If your primary focus is high-purity trace element or rare earth element (REE) analysis (e.g., using ICP-MS): Using an agate or alumina ceramic pulverizer is non-negotiable to avoid critical contamination from the grinding vessel.
- If you are prospecting for precious metals (e.g., gold): Standard preparation is likely to fail. You must use specialized methods like screen fire assay or Bulk Leach Extractable Gold (BLEG) that are designed to handle the "nugget effect."
- If you are performing mineralogical analysis (e.g., XRD): You must be cautious of over-grinding, which can damage crystal structures and render the results less accurate.
Meticulous sample preparation is the non-negotiable foundation upon which all reliable geochemical data is built.
Summary Table:
| Step | Purpose | Key Equipment |
|---|---|---|
| Initial Cleaning & Crushing | Remove contaminants, reduce size | Jaw Crusher |
| Splitting | Create a representative subsample | Riffle Splitter |
| Pulverizing | Achieve fine, homogeneous powder | Ring Mill (Agate, Alumina, Tungsten Carbide) |
Achieve reliable geochemical data with KINTEK's precision lab equipment.
Our robust jaw crushers, sample splitters, and high-purity pulverizing mills (including agate, alumina, and tungsten carbide options) are designed to prevent contamination and ensure your rock samples are perfectly prepared for XRF, ICP-MS, and other analytical techniques.
Contact our experts today to discuss the ideal sample preparation solution for your laboratory's specific needs.
Visual Guide
Related Products
- Laboratory Grinding Mill Mortar Grinder for Sample Preparation
- Laboratory Ball Mill Jar Mill with Metal Alloy Grinding Jar and Balls
- Stainless Steel Laboratory Ball Mill for Dry Powder and Liquid with Ceramic Polyurethane Lining
- Laboratory Four-Body Horizontal Jar Mill
- Laboratory Sealed Hammer Crusher for Efficient Sample Preparation
People Also Ask
- What are the lab safety rules for heating substances? Essential Protocols to Prevent Accidents
- How much temperature can graphite withstand? Unlock its true potential up to 3000°C
- What are the advantages of a water bath in the laboratory? Ensure Gentle, Uniform Heating for Sensitive Samples
- What is the capacity of a plate and frame filter press? Understand the real-world throughput for your slurry.
- What is the effect of catalyst on pyrolysis? Control Reaction Pathways for Higher-Value Products
- What is an ultra-low temperature freezer? Protect Your Most Valuable Biological Samples
- How long should a furnace take to warm up? Understanding the Normal 1-3 Minute Ignition Sequence
- What is the function of KBr? A Key Tool for High-Quality FTIR Analysis of Solids



















