To be precise, a muffle furnace is the instrument used to determine the ash content of a material sample; the furnace itself does not have an ash content. The procedure involves weighing a sample, placing it in the furnace to burn away all organic components at high temperatures, and then weighing the remaining inorganic residue, known as ash. This process, called ash analysis or "ashing," quantifies the non-combustible filler or mineral content within your sample.
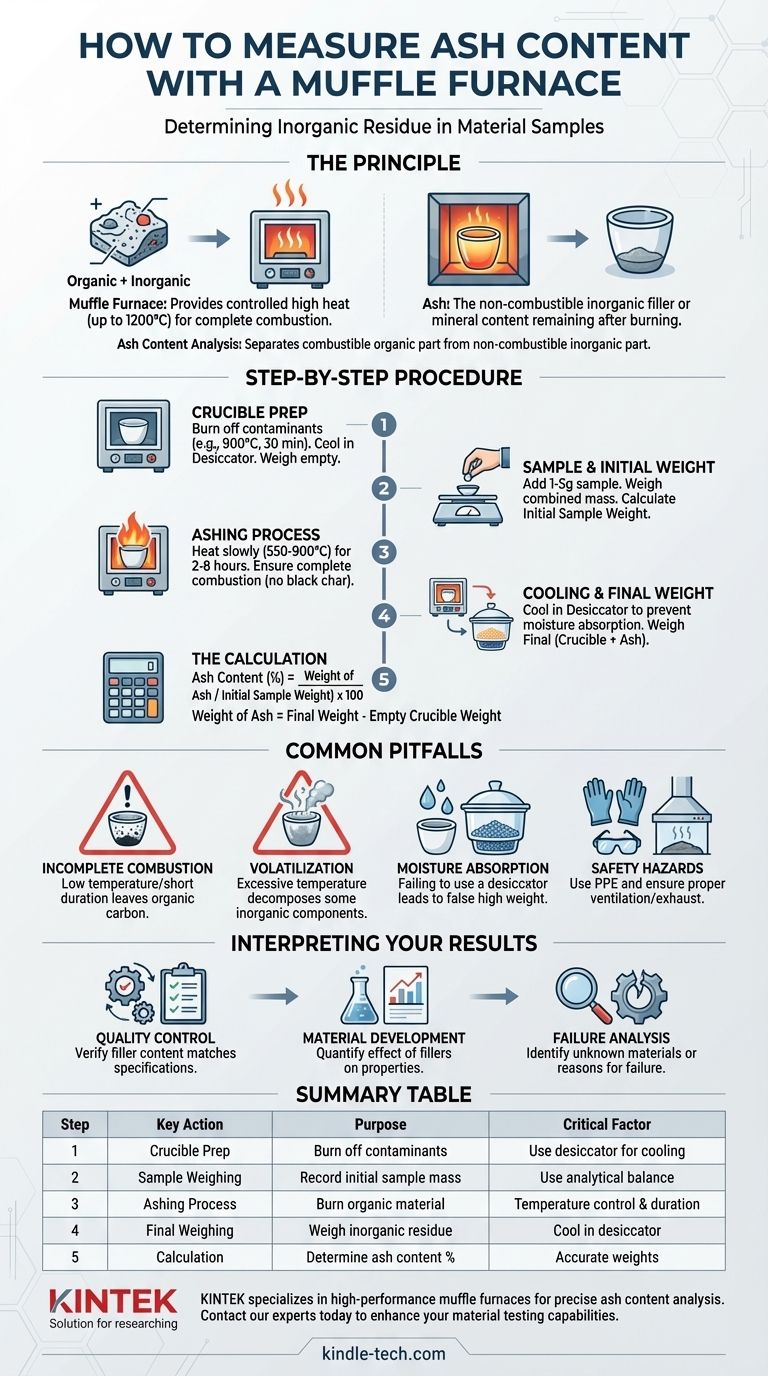
Ash content analysis is a fundamental gravimetric technique used in material science and quality control. It separates the combustible organic part of a material from the non-combustible inorganic part (the ash) through controlled high-temperature oxidation, providing a critical measure of filler content and material purity.

The Principle: Isolating the Inorganic Residue
The core purpose of ash analysis is to measure the weight percentage of inorganic materials present in a sample. These are often fillers added to a polymer or rubber to modify its properties or reduce cost.
What is "Ash"?
In this context, ash is the inorganic residue that remains after a material is completely burned. This can include mineral fillers like glass fibers, calcium carbonate, talc, or silica, as well as metallic salts and oxides.
How a Muffle Furnace Works
A muffle furnace is an oven that can reach very high, precisely controlled temperatures, often up to 1200°C (2192°F). It provides a uniform, high-heat environment that ensures the complete combustion of the organic polymer or rubber matrix, leaving only the stable inorganic ash behind.
Step-by-Step Procedure for Accurate Measurement
Following a rigorous procedure is critical for obtaining repeatable and accurate results. The essential tools are a muffle furnace, a high-precision analytical balance, porcelain crucibles, and a desiccator.
Step 1: Crucible Preparation
Before introducing your sample, the crucible must be prepared. Place the empty, clean porcelain crucible in the muffle furnace at the intended test temperature (e.g., 900°C) for about 30 minutes.
This step burns off any residual moisture or contaminants on the crucible itself. Afterwards, transfer the hot crucible to a desiccator to cool to room temperature without absorbing atmospheric moisture, then weigh it precisely on an analytical balance. This is your "empty crucible weight."
Step 2: Sample Preparation and Initial Weighing
Cut a small, representative portion of your material, typically 1-5 grams. Place this sample into the pre-weighed crucible.
Record the combined weight of the crucible and the sample using the analytical balance. By subtracting the empty crucible weight, you get the "initial sample weight."
Step 3: The Ashing Process
Place the crucible containing the sample into the cool or slightly warm muffle furnace. Slowly ramp the temperature up to the target, which can range from 550°C to 900°C depending on the material and the specific standard being followed (e.g., ASTM D2584 for polymers).
Holding the sample at this peak temperature ensures complete combustion of the organic components. The duration can range from 2 to 8 hours, until all the black carbon char has disappeared, leaving only a light-colored ash.
Step 4: Cooling and Final Weighing
Once the ashing is complete, turn off the furnace and allow it to cool significantly before carefully removing the crucible. Immediately place the hot crucible into a desiccator.
The desiccator contains a drying agent and provides a moisture-free environment for the crucible to cool to room temperature. This is a critical step, as many ash residues are hygroscopic and will absorb moisture from the air, artificially increasing their weight.
Once cooled, weigh the crucible containing the ash. This gives you the "final weight (crucible + ash)."
Step 5: The Calculation
The calculation for ash content is straightforward. First, determine the weight of the ash residue:
- Weight of Ash = (Final weight [crucible + ash]) - (Empty crucible weight)
Then, calculate the percentage of ash relative to the initial sample:
- Ash Content (%) = (Weight of Ash / Initial Sample Weight) x 100
Common Pitfalls to Avoid
Accurate ash analysis requires avoiding several common sources of error.
Incomplete Combustion
If the temperature is too low or the duration is too short, some organic carbon may remain, appearing as black specks in the residue. This will falsely inflate the measured ash content. Ensure the final residue is a uniform, light color.
Volatilization of Ash Components
Conversely, an excessively high temperature can cause certain inorganic components to decompose or volatilize. For example, calcium carbonate (CaCO₃) can decompose to calcium oxide (CaO) above 825°C, releasing CO₂ and causing a loss of mass. Know your material's composition to select the correct ashing temperature.
Moisture Absorption
Failing to use a desiccator for cooling is a frequent mistake. The ash residue will immediately begin absorbing moisture from the air once it cools below 100°C, leading to an inaccurately high final weight.
Safety Hazards
Always use proper personal protective equipment (PPE), including heat-resistant gloves and safety glasses, when operating a muffle furnace. Ensure the furnace is located in a well-ventilated area or under a fume hood to safely exhaust the products of combustion.
Interpreting Your Results for a Clear Purpose
The ash content percentage is not just a number; it's a key indicator of material composition and quality.
- If your primary focus is quality control: Use this method to verify that the filler content of incoming raw materials or finished products matches the required specification.
- If your primary focus is material development: Use ash content to quantify the effect of different filler types and loadings on the physical and mechanical properties of a new composite.
- If your primary focus is failure analysis or reverse-engineering: Use the ash percentage as a crucial piece of data to help identify an unknown material or understand why a component may have failed to perform as expected.
Ultimately, mastering this fundamental test provides a clear and reliable window into the true composition of your materials.
Summary Table:
| Step | Key Action | Purpose | Critical Factor |
|---|---|---|---|
| 1 | Crucible Preparation | Burn off contaminants, obtain tare weight | Use desiccator for cooling |
| 2 | Sample Weighing | Record initial sample mass (1-5g) | Use analytical balance |
| 3 | Ashing Process | Burn organic material at 550-900°C | Temperature control & duration |
| 4 | Final Weighing | Weigh inorganic residue | Cool in desiccator to prevent moisture absorption |
| 5 | Calculation | Determine ash content percentage | Formula: (Ash Weight / Sample Weight) × 100 |
Need precise ash content analysis for your materials? KINTEK specializes in high-performance muffle furnaces and laboratory equipment that deliver accurate, repeatable results for quality control, material development, and failure analysis. Our solutions ensure complete combustion, precise temperature control, and reliable data for polymers, rubbers, and composites. Contact our experts today to find the perfect furnace for your lab's needs and enhance your material testing capabilities.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the safety rules for all heating process in the laboratory? A Guide to Preventing Accidents
- What does 'sintered' mean and why is it important to understand? Unlock Advanced Materials & Manufacturing
- What is the debinding process? A Guide to Critical Binder Removal for MIM & 3D Printing
- How does heat treatment affect surface roughness? Minimize Surface Degradation for Precision Parts
- What are the classification of refractory materials? A Guide to Chemical and Thermal Selection



















