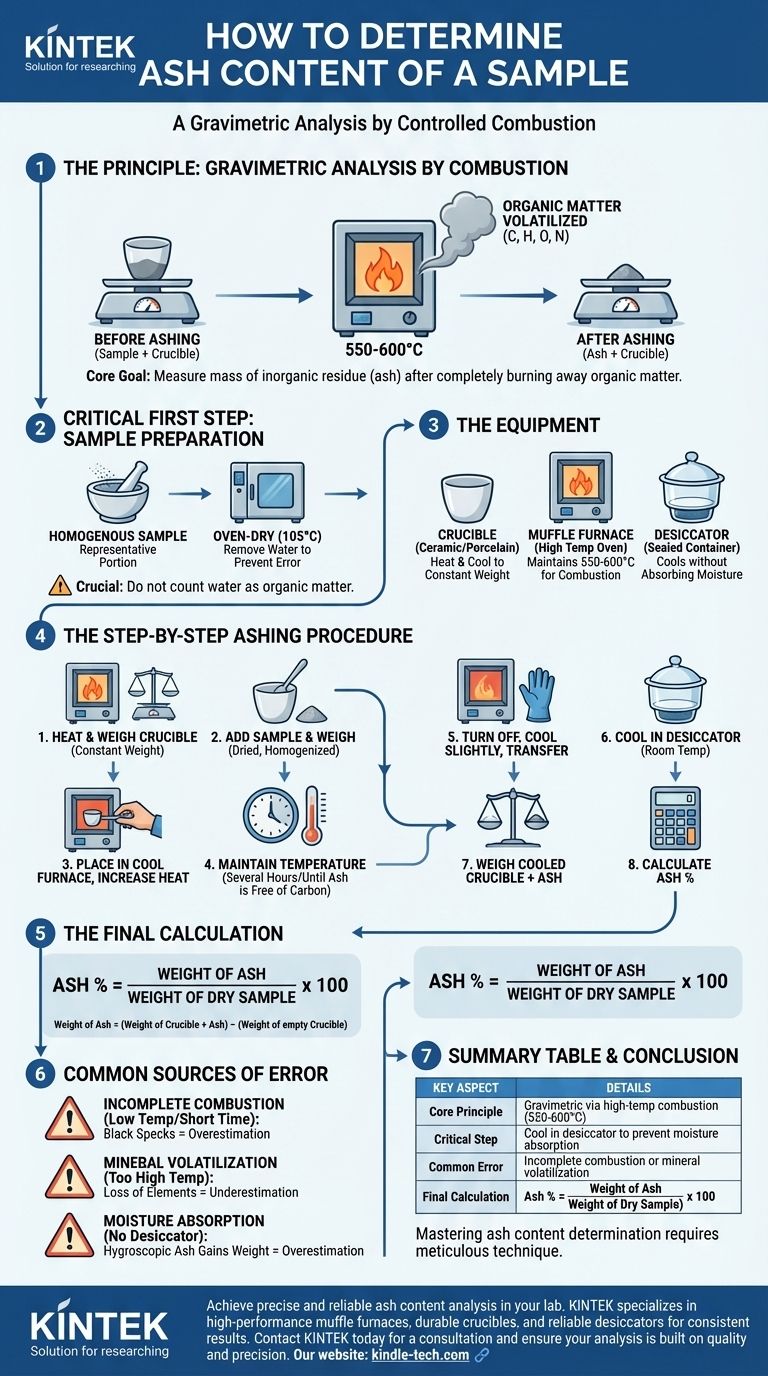
At its core, determining the ash content of a sample is a straightforward process of controlled combustion. The method involves heating a sample at a high temperature to completely burn away all organic matter, leaving behind only the inorganic, non-combustible residue. This residue, known as ash, is then weighed to quantify the mineral content of the original material.
The determination of ash is a gravimetric analysis technique, meaning it relies on measurement by mass. The fundamental goal is to measure the weight of a sample before and after complete incineration to find the mass of the inorganic residue that remains.

The Principle: Gravimetric Analysis by Combustion
The entire procedure is often referred to as "dry ashing" or "loss-on-ignition." It operates on the simple principle that high heat will decompose and volatilize organic substances (carbon, hydrogen, oxygen, nitrogen) while leaving stable inorganic oxides and salts behind.
The Critical First Step: Sample Preparation
Accuracy begins before the sample ever sees heat. The sample must be homogenous to ensure the small portion being tested is representative of the whole batch.
Crucially, the sample is typically oven-dried at a lower temperature (e.g., 105°C) before ashing. This step removes water, which would otherwise evaporate during ashing and be incorrectly counted as part of the organic matter, falsely inflating the final ash percentage.
The Equipment: Crucible, Furnace, and Desiccator
The primary tool for this process is a muffle furnace, an insulated oven capable of reaching and maintaining the high temperatures required for complete combustion, typically between 550°C and 600°C.
The sample is held in a crucible, a ceramic or porcelain cup designed to withstand extreme thermal shock. Before use, this crucible must be heated to the ashing temperature, cooled, and weighed until it reaches a constant weight, ensuring any residue or moisture on the crucible itself doesn't affect the final measurement.
After heating, the hot crucible is placed in a desiccator. This is a sealed container with a drying agent (a desiccant) that allows the crucible and its contents to cool to room temperature without absorbing moisture from the air.
The Step-by-Step Ashing Procedure
- Heat a clean, empty crucible in a muffle furnace, cool it in a desiccator, and weigh it. Repeat this cycle until a constant weight is achieved.
- Add a precisely weighed amount of the dried, homogenized sample to the pre-weighed crucible.
- Place the crucible with the sample into the cool muffle furnace. Gradually increase the temperature to the target (e.g., 550°C) to avoid spattering.
- Maintain the target temperature for several hours (typically 2-4 hours, or until the ash is visibly free of black carbon particles).
- Turn off the furnace, open the door slightly to allow for slow initial cooling, and then transfer the hot crucible to a desiccator.
- Allow the crucible to cool completely to room temperature inside the desiccator.
- Weigh the cooled crucible containing the ash.
- Calculate the percentage of ash using the final weights.
The Final Calculation
The calculation is a simple expression of the residue's weight as a percentage of the initial sample's weight.
Ash % = (Weight of Ash / Weight of Dry Sample) x 100
Where Weight of Ash = (Weight of Crucible + Ash) - (Weight of empty Crucible).
Understanding the Trade-offs and Sources of Error
While the procedure is simple in principle, meticulous technique is required to avoid significant errors. Understanding these potential pitfalls is key to generating reliable data.
Incomplete Combustion
If the temperature is too low or the ashing time is too short, not all carbon will be burned off. This is often visible as black specks in the ash residue. The remaining carbon will add weight, leading to an overestimation of the true ash content.
Volatilization of Minerals
Conversely, if the temperature is too high, some inorganic salts and elements can be lost through volatilization or decomposition. Elements like chlorine, sulfur, sodium, and potassium can be partially lost, leading to an underestimation of the true ash content. The standard 550-600°C range is a compromise to minimize both incomplete combustion and mineral loss.
Moisture Absorption
Ash residue is often hygroscopic, meaning it readily absorbs moisture from the air. Failing to use a desiccator for cooling will cause the ash to gain weight from atmospheric water, resulting in an overestimation of the ash content. This is one of the most common procedural errors.
Making the Right Choice for Your Goal
The rigor of your procedure should match your analytical objective. A few targeted adjustments can ensure your results are fit for purpose.
- If your primary focus is regulatory compliance or QC: Adhere strictly to a standardized method (e.g., AOAC for food, ASTM for materials) and meticulously document every step, especially temperature, duration, and achieving constant weights.
- If your primary focus is routine process control: Consistency is more important than absolute accuracy. Ensure your lab's internal procedure is followed identically every time to track trends and deviations reliably.
- If you are troubleshooting inconsistent results: Your first checks should be visual inspection of the ash for black particles (incomplete combustion) and re-evaluating your cooling and weighing protocol to eliminate moisture absorption as a variable.
Ultimately, mastering ash content determination is a testament to careful, precise laboratory technique.
Summary Table:
| Key Aspect | Details |
|---|---|
| Core Principle | Gravimetric analysis via high-temperature combustion (550-600°C) |
| Primary Equipment | Muffle furnace, crucible, desiccator, analytical balance |
| Critical Step | Cooling ash in a desiccator to prevent moisture absorption |
| Common Error | Incomplete combustion (overestimation) or mineral volatilization (underestimation) |
| Final Calculation | Ash % = (Weight of Ash / Weight of Dry Sample) x 100 |
Achieve precise and reliable ash content analysis in your lab.
Accurate determination of ash and mineral content is critical for quality control, compliance, and research. The process relies on robust equipment and meticulous technique to avoid common errors like moisture absorption or incomplete combustion.
KINTEK specializes in supplying the precise laboratory equipment you need for this essential procedure, including high-performance muffle furnaces for consistent high-temperature ashing, durable crucibles, and reliable desiccators to ensure your results are accurate and reproducible.
Let us help you enhance your lab's capabilities. Whether you're setting up a new QC protocol or optimizing an existing one, our experts can provide the right tools and support.
Contact KINTEK today for a consultation and ensure your ash content analysis is built on a foundation of quality and precision.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How is melting point affected by heating rate? Avoid Inaccurate Measurements in Your Lab
- What temperature does molten steel melt? Understand the Melting Range, Not a Single Point
- Why is the metal melting temperature important? The Key to Manufacturing & Performance
- What is ramp rate and how does that affect a melting point measurement? Master the Key to Accurate Thermal Analysis
- What affects melting range? Understand the Critical Role of Purity and Structure



















