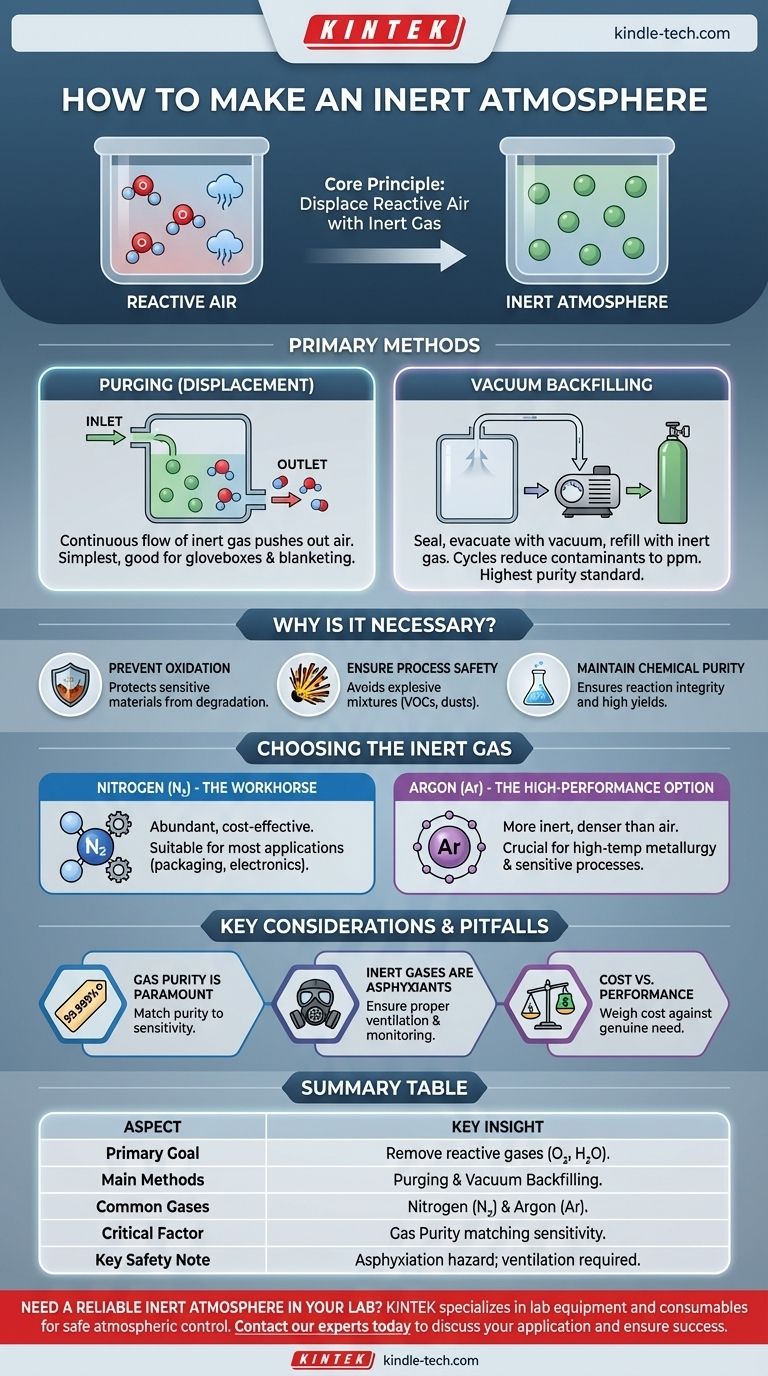
In practice, an inert atmosphere is created by physically displacing the reactive air inside a contained space and replacing it with a non-reactive gas. The two primary methods for achieving this are purging, which involves flowing inert gas to push the air out, and vacuum backfilling, where the air is first removed with a vacuum pump and the space is then refilled with the inert gas.
The fundamental goal of creating an inert atmosphere is not just to add a special gas, but to actively remove reactive gases—primarily oxygen and water vapor. This protects sensitive materials and processes from unwanted chemical reactions, degradation, and safety hazards.

The Core Principle: Why Inerting is Necessary
Creating an inert atmosphere, or "inerting," is a foundational technique in science and industry. It is used whenever the standard air we breathe would interfere with a desired outcome.
To Prevent Oxidation and Degradation
Many materials, from fine chemicals and pharmaceuticals to metals at high temperatures, react with oxygen. This process, called oxidation, can degrade product quality, change chemical properties, or create impurities. An inert atmosphere eliminates the oxygen, effectively pausing these degradation pathways.
To Ensure Process Safety
Volatile organic compounds (VOCs) or fine combustible dusts can form an explosive mixture with the oxygen in the air. By replacing the oxygen with an inert gas, you can bring the oxygen concentration below the lower explosive limit (LEL), preventing fires and explosions in reactors, storage tanks, and transfer lines.
To Maintain Chemical Purity
In sensitive chemical synthesis, such as in organometallic chemistry, reactants can be destroyed by trace amounts of oxygen or water. An inert atmosphere is not just a suggestion but a requirement to ensure the reaction proceeds as intended and achieves a high yield of the desired product.
Key Methods for Creating an Inert Atmosphere
The method you choose depends on the geometry of your container and the level of purity required.
Method 1: Purging (Displacement)
Purging is the simplest method. It involves introducing a continuous flow of inert gas into a vessel, typically through an inlet at one end, while allowing the displaced air to exit through an outlet at the other.
This works就像 trying to clear a bottle of smoky air by blowing clean air into it. Eventually, the smoke is diluted and pushed out. This method is common for gloveboxes, Schlenk lines, and blanketing the headspace of storage tanks.
Method 2: Vacuum Backfilling
For the highest level of purity, vacuum backfilling is superior. The process involves sealing the chamber, using a vacuum pump to evacuate nearly all of the air, and then refilling the chamber with high-purity inert gas.
This cycle of evacuating and refilling can be repeated multiple times (typically 3-5 cycles) to reduce trace atmospheric contaminants to parts-per-million (ppm) levels or lower. This is the standard metoda for highly sensitive applications.
Choosing the Right Inert Gas
While several gases are non-reactive, two dominate nearly all applications due to their availability and properties.
Nitrogen (N₂): The Workhorse
Nitrogen gas is the most common choice. It is industrially separated from air, making it abundant and cost-effective. It is suitable for the vast majority of applications, including food packaging, electronics manufacturing, and general chemical blanketing.
Argon (Ar): The High-Performance Option
Argon is more inert than nitrogen and is crucial for processes where nitrogen could still react, such as in high-temperature metallurgy where metal nitrides might form. Argon is also denser than air, allowing it to form a stable "blanket" over sensitive materials in an open-topped container, displacing the lighter air upwards.
Understanding the Trade-offs and Pitfalls
Successfully implementing an inert atmosphere requires attention to detail beyond just picking a gas.
Gas Purity is Paramount
The term "inert gas" is only half the story. The purity of the gas is what truly matters. Using a cylinder of "industrial grade" nitrogen漏洞 with 100 ppm of oxygen will not protect a reaction that is sensitive to 5 ppm of oxygen. Always match the gas purity (e.g., 99.999% or "five-nines") to the sensitivity of your application.
Inert Gases Are Asphyxiants
A critical safety consideration is that inert gases displace oxygen. Any leak in a poorly ventilated area can create an oxygen-deficient atmosphere, posing a severe asphyxiation hazard. Always ensure proper ventilation and use oxygen monitors where appropriate.
Cost vs. Performance
There is a direct cost trade-off. Nitrogen is significantly cheaper than argon. You must weigh the added cost of argon against the genuine need for its higher level of inertness. For most applications, nitrogen is the more practical and economical choice.
Selecting the Right Approach for Your Application
Your final decision should be guided by your specific technical and safety requirements.
- If your primary focus is general-purpose storage or preventing basic oxidation: Purging with a continuous, low-flow stream of standard purity nitrogen is effective and economical.
- If your primary focus is high-temperature metallurgy or highly sensitive chemistry: Vacuum backfilling with high-purity argon is the required standard to prevent unwanted side reactions.
- If your primary focus is fire and explosion prevention in a large vessel: Blanketing the headspace with nitrogen to keep the oxygen level below the explosive limit is the critical safety measure.
- If your primary focus is bench-scale air-sensitive chemistry: A Schlenk line or glovebox नमी purging with nitrogen or argon is the standard laboratory setup.
Mastering atmospheric control is the key to ensuring the safety, purity, and reliability of your sensitive processes.
Summary Table:
| Aspect | Key Insight |
|---|---|
| Primary Goal | Remove reactive gases (oxygen, water vapor) to protect materials and processes. |
| Main Methods | Purging (for simplicity) and Vacuum Backfilling (for high purity). |
| Common Gases | Nitrogen (cost-effective) and Argon (high-performance). |
| Critical Factor | Gas purity must match the sensitivity of the application. |
| Key Safety Note | Inert gases are asphyxiants; ensure proper ventilation and monitoring. |
Need to implement a reliable inert atmosphere in your lab?
KINTEK specializes in lab equipment and consumables, providing the precise tools and expert guidance you need for safe and effective atmospheric control. Whether you're working with sensitive chemical synthesis, high-temperature processes, or require explosion prevention, we can help you select the right solution—from gas purification systems to vacuum pumps and specialized chambers.
Contact our experts today to discuss your specific application and ensure the purity, safety, and success of your work.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes
- What is an inert condition? A Guide to Preventing Fires and Explosions
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety



















