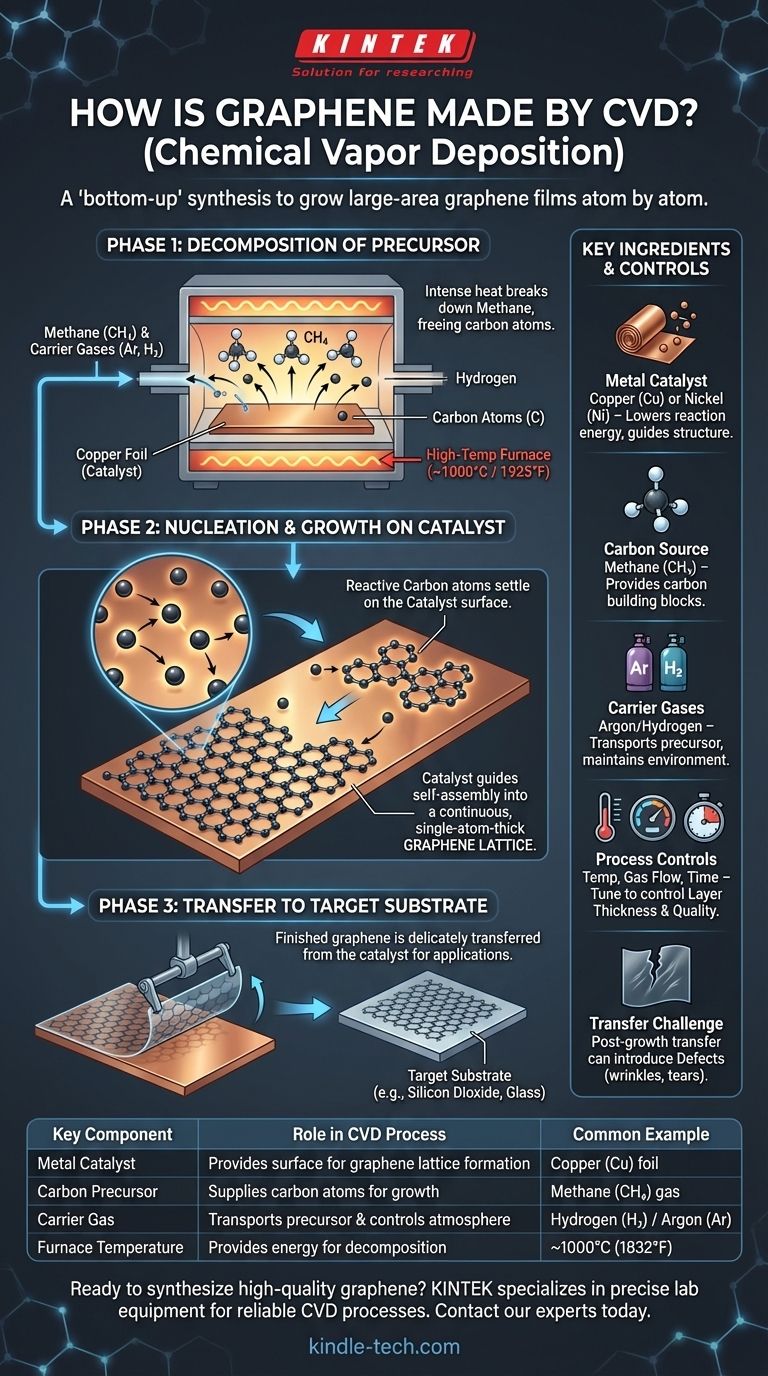
To create graphene via Chemical Vapor Deposition (CVD), a metal substrate like copper foil is placed inside a high-temperature furnace, typically around 1000°C. A carbon-containing gas, most often methane, is then introduced into the chamber. The intense heat breaks this gas down, freeing carbon atoms that then settle onto the surface of the metal catalyst, self-assembling into a continuous, single-atom-thick sheet of graphene.
Chemical Vapor Deposition is a "bottom-up" synthesis method that effectively "grows" a large-area graphene film atom by atom. The process relies on the thermal decomposition of a carbon gas onto a catalytic metal surface, with precise control over the environment being the key to producing a high-quality, uniform film.

The Fundamentals of the CVD Process
To understand how CVD works, it's best to think of it as a controlled construction project on an atomic scale. It is a "bottom-up" approach, meaning you are building the material from its fundamental atomic components, not carving it from a larger block.
The Key Ingredients for Graphene Growth
The success of the CVD process depends on four critical components working in concert inside a specialized reactor.
- Metal Substrate (Catalyst): A metal foil, typically copper (Cu) or nickel (Ni), acts as the foundation. Its primary role is to be a catalyst, lowering the energy required for the reaction and providing an ideal surface for carbon atoms to arrange themselves into the graphene structure.
- Carbon Source (Precursor): This is the "building block" material. A hydrocarbon gas, most commonly methane (CH4), is used because it readily decomposes at high temperatures to provide the necessary carbon atoms.
- Carrier Gases: Inert gases like Argon (Ar) or reactive gases like Hydrogen (H2) are used. They help transport the precursor gas into the reaction chamber and maintain a controlled atmospheric environment.
- High-Temperature Environment: The entire process takes place in a furnace at extreme temperatures, often around 1000°C (1925°F). This heat is essential to provide the energy needed to break the chemical bonds in the precursor gas.
A Step-by-Step Breakdown of Graphene Synthesis
The creation of a graphene film can be broken down into two primary phases: the decomposition of the carbon source and the formation of the graphene lattice.
Phase 1: Decomposition of the Precursor
The process begins by heating the metal substrate within the furnace. Once the target temperature is reached, the methane and carrier gases are introduced into the chamber.
The intense heat triggers pyrolysis, causing the methane (CH4) molecules to decompose. This chemical reaction breaks them down into their constituent carbon atoms (C) and hydrogen.
Phase 2: Nucleation and Growth on the Catalyst
The freed carbon atoms are now highly reactive and mobile on the hot metal surface. They begin to bind to the copper substrate and, more importantly, to each other.
This is where the catalytic nature of the copper is crucial. It guides the carbon atoms to arrange themselves into the stable, honeycomb-like hexagonal lattice that defines graphene. This growth starts at multiple points, forming small "islands" of graphene that expand and eventually merge into a continuous, single-atom-thick film covering the substrate.
Phase 3: Transfer to a Target Substrate
Once the graphene film has grown, the system is cooled. However, the graphene is still on the metal foil where it was grown. For most electronic or optical applications, it must be moved.
This involves a delicate transfer process, where the graphene sheet is carefully lifted off the copper foil and placed onto a different, non-conductive substrate like silicon dioxide or glass.
Understanding the Trade-offs and Controls
While CVD is a powerful method for producing large-area graphene, it is a process with critical variables and inherent challenges that determine the quality of the final product.
Controlling Layer Thickness
The number of graphene layers (e.g., single-layer vs. bilayer) is not random. It is controlled by carefully tuning the process parameters. Factors like the gas flow rate, reaction temperature, and the duration of exposure to the carbon source all influence the final thickness.
The Challenge of Transfer
The post-growth transfer process is the primary source of defects in CVD graphene. Wrinkles, tears, and chemical residues from the transfer can be introduced into the film, compromising its pristine structure and exceptional electronic properties. A perfect growth process can be undermined by a poor transfer.
The Impact of the Substrate
The choice of metal catalyst is significant. Copper is the most common choice for producing high-quality, single-layer graphene. Other substrates, like nickel, have different properties that can lead to multi-layer graphene growth. The nature of the substrate directly impacts the growth mechanism.
Making the Right Choice for Your Goal
Understanding the CVD process allows you to tailor your approach based on the desired outcome.
- If your primary focus is large-area, uniform films: CVD is the industry-standard method precisely because it excels at producing continuous graphene sheets over large areas, far beyond what is possible with other techniques.
- If your primary focus is ultimate electronic quality: Your attention must be on perfecting the post-growth transfer process, as this is the step most likely to introduce performance-limiting defects.
- If your primary focus is process control and repeatability: Mastering the precise interplay between temperature, gas flow rates, and growth time is the key to reliably tuning the properties of your graphene, such as layer count.
By understanding these fundamental steps and control levers, you can effectively harness the CVD process to create high-quality graphene for advanced materials science and next-generation device engineering.
Summary Table:
| Key Component | Role in CVD Process | Common Example |
|---|---|---|
| Metal Catalyst | Provides surface for carbon atoms to form graphene lattice | Copper (Cu) foil |
| Carbon Precursor | Supplies carbon atoms for graphene growth | Methane (CH₄) gas |
| Carrier Gas | Transports precursor and controls atmosphere | Hydrogen (H₂) / Argon (Ar) |
| Furnace Temperature | Provides energy for precursor decomposition | ~1000°C (1832°F) |
Ready to synthesize high-quality graphene in your lab? KINTEK specializes in the precise lab equipment and consumables needed for reliable CVD processes. From high-temperature furnaces to catalytic substrates, our solutions help you achieve uniform, large-area graphene films with exceptional control. Contact our experts today to discuss how we can support your advanced materials research and development.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
People Also Ask
- What is PECVD in semiconductor? Enable Low-Temperature Thin Film Deposition for ICs
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings
- What is the process of vacuum vapor deposition? Mastering CVD and PVD Thin-Film Coating
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition



















