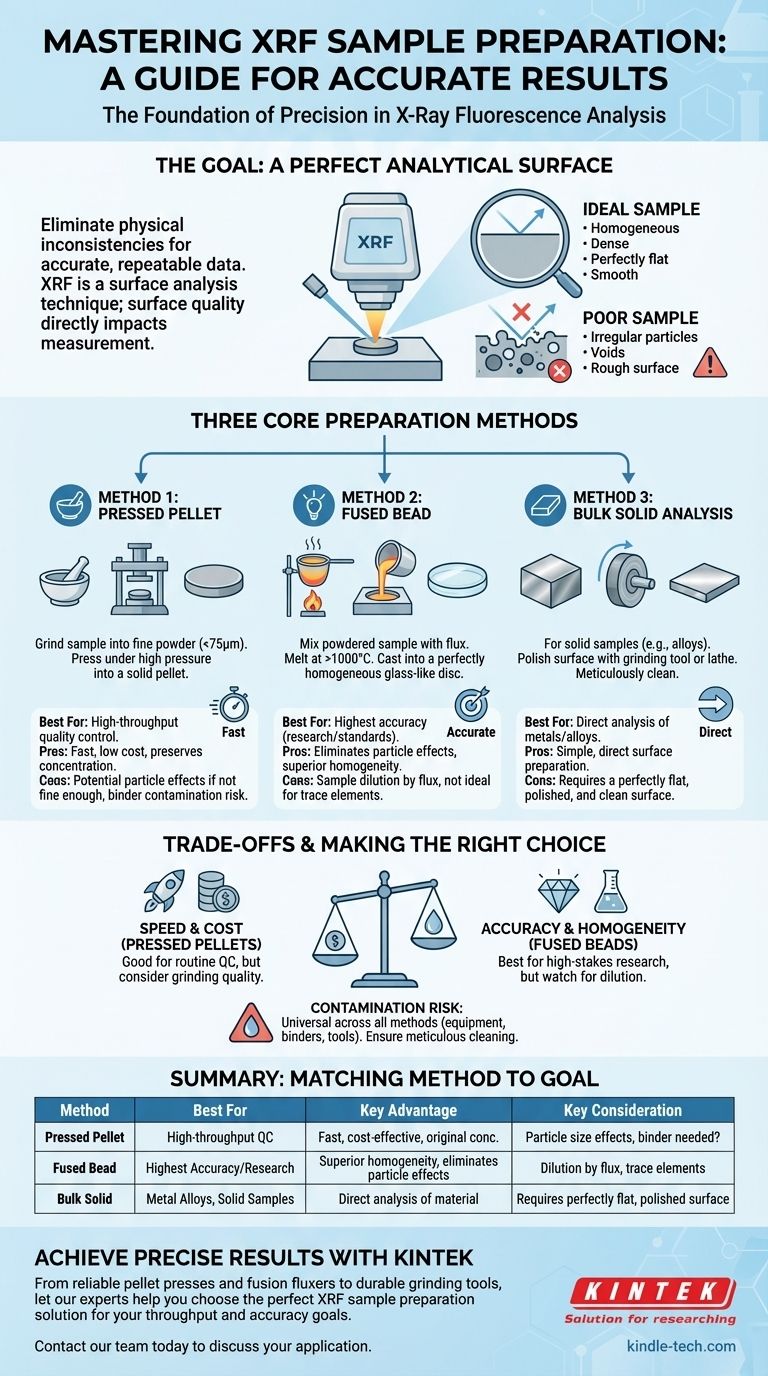
To prepare a sample for XRF analysis, you must transform it into a robust, homogeneous solid with a perfectly flat surface. The most common methods involve either grinding the material into a fine powder and compressing it into a pressed pellet, or mixing it with a flux and melting it into a fused glass-like bead.
The fundamental goal of any XRF sample preparation is to eliminate physical inconsistencies. By creating a perfectly uniform and flat surface, you ensure that the instrument measures the sample's true elemental composition, not the misleading effects of particle size, voids, or surface roughness.

The Principle: Why Preparation is Critical
XRF is a surface analysis technique. The instrument bombards the sample's surface with X-rays and measures the secondary X-rays that are emitted back. Inaccurate results are almost always caused by a poorly prepared sample surface.
The Impact of an Inconsistent Surface
An ideal sample is perfectly homogeneous, meaning it has a uniform composition throughout. It must also be representative of the bulk material it came from.
If a sample is composed of large, irregular particles, the X-ray beam can be scattered unpredictably. Voids between particles and a rough surface can also alter the signal, leading to significant analytical errors.
The Goal: A Perfect Analytical Surface
All preparation methods aim to create a surface that is dense, flat, and free of physical variations. This minimizes analytical errors and ensures the data you collect is both accurate and repeatable.
Core Preparation Methods Explained
The right method depends on your sample type, the accuracy required, and the resources available.
Method 1: The Pressed Pellet
This is the most popular method due to its speed, low cost, and excellent results for many sample types.
The process involves crushing and grinding the sample into a very fine powder, typically smaller than 75 micrometers. This powder is then placed in a die and pressed under high pressure to form a dense, solid pellet.
Method 2: The Fused Bead
This method offers the highest level of accuracy by creating an almost perfectly homogeneous sample.
The powdered sample is mixed with a lithium borate flux and heated in a crucible to over 1000°C. The mixture melts into a molten liquid, which is then cast into a mold to cool into a perfectly smooth, glass-like disc.
Method 3: Bulk Solid Analysis
For solid samples like metal alloys, preparation is simpler but still crucial.
The goal is to create a flat, clean measurement surface. This is typically achieved by polishing the sample with a grinding tool or using a lathe for softer metals. The surface must then be cleaned to remove any residue.
Understanding the Trade-offs
No single method is perfect for every situation. Understanding the advantages and disadvantages is key to making the right choice.
Pressed Pellets: Speed vs. Particle Effects
Pressed pellets are fast and preserve the sample's original concentration. However, if the material is not ground finely or uniformly enough, residual particle size effects can still introduce minor inaccuracies. If the powder doesn't bind well, a binder is needed, which can be a source of contamination.
Fused Beads: Accuracy vs. Dilution
Fusion completely eliminates particle size effects, yielding superior accuracy. The major trade-off is dilution. The flux significantly dilutes the sample, which can make it difficult to measure elements present at very low, trace-level concentrations.
Contamination: The Universal Risk
Contamination is a risk across all methods. The grinding equipment, binders used for pellets, and even shared cleaning files for bulk solids can introduce external elements into your sample, skewing the final results.
Making the Right Choice for Your Goal
Your analytical objective should guide your preparation strategy.
- If your primary focus is high-throughput quality control: The pressed pellet method offers the best balance of speed, cost, and reliable results.
- If your primary focus is the highest possible accuracy for research or standard creation: The fused bead method is the superior choice, as it removes the physical variables that can compromise data quality.
- If your primary focus is analyzing a solid metal or alloy: Direct surface preparation through polishing and meticulous cleaning is the correct and most efficient approach.
Ultimately, mastering these preparation principles is the key to generating consistently accurate and defensible XRF data.
Summary Table:
| Method | Best For | Key Advantage | Key Consideration |
|---|---|---|---|
| Pressed Pellet | High-throughput quality control | Fast, cost-effective, preserves original concentration | Potential for minor particle effects if not ground finely |
| Fused Bead | Highest accuracy for research/standards | Eliminates particle effects, superior homogeneity | Sample dilution by flux can mask trace elements |
| Bulk Solid Analysis | Metal alloys, solid samples | Direct analysis of the material | Requires a perfectly flat, polished, and clean surface |
Achieve precise and reliable XRF analysis in your lab. The right sample preparation is the foundation of accurate data. KINTEK specializes in high-quality lab equipment and consumables for all your XRF needs, from reliable pellet presses and fusion fluxers to durable grinding tools. Let our experts help you choose the perfect solution for your throughput and accuracy goals. Contact our team today to discuss your application and ensure your results are consistently defensible.
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- XRF Boric Acid Lab Powder Pellet Pressing Mold for Laboratory Use
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- What does the working principle of a hydraulic press depend on? Harness Pascal's Law for Immense Force
- What is the working of a hydraulic process? Harness Pascal's Law for Immense Force
- How does temperature affect forging? Master Hot, Cold, and Warm Forging for Optimal Results
- How can a laboratory hydraulic press be utilized for the cold welding of silver nanowire junctions? Achieve Low Resistance
- Why is a high-pressure laboratory hydraulic press required for pelletizing solid-state battery components?
- Why is a laboratory hydraulic press preferred over sintering for sulfide electrolyte anode frameworks? (LPS)
- Can a hydraulic press break a diamond? Yes, and here’s why hardness isn't strength.
- What is the procedure for XRF? A Step-by-Step Guide to Accurate Elemental Analysis



















