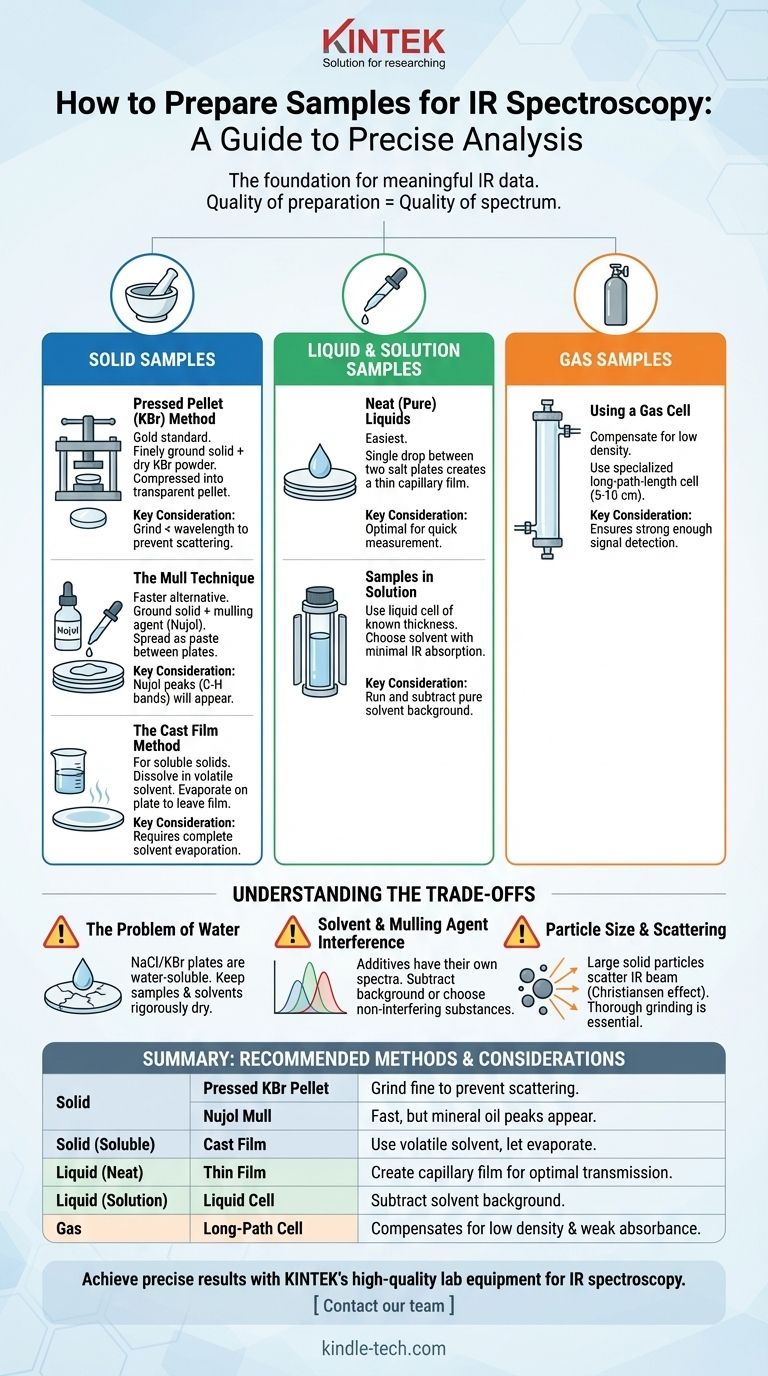
The right preparation method for IR spectroscopy depends entirely on the physical state of your sample—whether it is a solid, liquid, or gas. For liquids, a thin film between salt plates is common. For solids, techniques include creating a pressed KBr pellet, making a mull, or casting a film from a solution. Gas samples require a specialized long-path-length cell to ensure a strong enough signal is detected.
Your goal in sample preparation is not just to get the material into the spectrometer. It is to make the sample sufficiently transparent to the infrared beam while minimizing any interfering signals from solvents, moisture, or the preparation medium itself.

The Foundation: Why Sample Preparation Matters
Proper sample preparation is the most critical step in obtaining a meaningful IR spectrum. The quality of your data is a direct result of the quality of your sample preparation.
The Cost of Poor Preparation
If a sample is prepared incorrectly, the resulting spectrum can be misleading. Peaks can be too broad, their intensities can be wrong, or the entire baseline might be sloped and unusable. This can lead to incorrect structural interpretations.
The Goal: A Clean, Analyzable Signal
An ideal sample allows enough IR radiation to pass through it (i.e., it isn't too concentrated) so that the detector can measure the absorbance accurately. The goal is to see sharp, well-defined peaks corresponding to the compound's functional groups, not artifacts from the preparation process.
Preparing Solid Samples
Solids cannot be analyzed directly and must be dispersed in a medium that is transparent to infrared radiation.
The Pressed Pellet (KBr) Method
This is often considered the gold standard for high-quality spectra of solid samples. The solid is finely ground and mixed with a dry, powdered alkali halide, most commonly potassium bromide (KBr).
The mixture is then placed into a pellet die and compressed under high pressure using a hydraulic press. This process sinters the KBr and sample into a small, transparent pellet that can be placed directly in the spectrometer's sample holder. The key is to grind the sample to a particle size smaller than the wavelength of the IR light to prevent scattering.
The Mull Technique
A mull is a faster, though often lower-quality, alternative to a KBr pellet. The solid sample is ground into a fine powder and then mixed with a few drops of a mulling agent, typically Nujol (mineral oil).
This creates a thick paste, which is then spread thinly between two salt plates (like NaCl or KBr). The oil reduces light scattering from the solid particles. The major drawback is that the Nujol itself has C-H stretching and bending bands that will appear in your spectrum.
The Cast Film Method
If your solid is soluble in a volatile solvent, you can prepare a cast film. You dissolve a small amount of the compound in a suitable solvent (like dichloromethane or acetone).
A drop of this solution is placed on a single salt plate, and the solvent is allowed to evaporate. This leaves behind a thin, solid film of your compound on the plate, which can then be analyzed.
Preparing Liquid and Solution Samples
Liquids are generally the easiest samples to prepare for IR analysis.
Neat (Pure) Liquids
For a pure liquid sample, a single drop is placed onto the surface of a salt plate. A second salt plate is placed on top, and the two are gently pressed together to create a very thin capillary film. The assembly is then placed in the spectrometer.
Samples in Solution
If you need to analyze a sample in solution, you will use a liquid sample cell. These cells have salt windows and a spacer of a known thickness (path length) that contains the solution.
It is critical to choose a solvent that has minimal absorption in the IR regions you are interested in. You must also run a background spectrum of the pure solvent in the same cell and subtract it from your sample's spectrum to remove the solvent's contribution.
Preparing Gas Samples
Gases have very low densities compared to liquids and solids, meaning they absorb much less infrared radiation.
Using a Gas Cell
To compensate for weak absorbance, gas samples are analyzed in a specialized gas cell with a long path length, typically between 5 and 10 centimeters. The cell is first evacuated, then filled with the gas sample to be analyzed. The cell has IR-transparent windows (usually KBr) at both ends.
Understanding the Trade-offs
Choosing a method involves balancing convenience with potential sources of error.
The Problem of Water
Most common IR optical materials, like NaCl and KBr plates, are alkali halides. They are highly soluble in water and will be fogged or destroyed by contact with wet samples or even moisture from the air. All samples and solvents must be rigorously dry.
Solvent and Mulling Agent Interference
Any substance you add to your sample—a solvent for a solution or Nujol for a mull—will have its own IR spectrum. You must either choose a substance with known, non-interfering peaks or mathematically subtract its spectrum from your data.
Particle Size and Scattering
For solid samples (pellets and mulls), particle size is critical. If the solid particles are too large, they will scatter the IR beam rather than absorb it. This scattering effect, known as the Christiansen effect, leads to a sloping baseline and distorted, asymmetric peak shapes, making the spectrum difficult to interpret. Thorough grinding is essential.
Making the Right Choice for Your Sample
Your decision should be guided by your sample's physical state and your analytical goal.
- If you have a pure liquid: Use the neat film method between two salt plates for a quick and easy measurement.
- If you have a solid sample: The KBr pellet method provides the highest quality spectrum, while the Nujol mull is a faster but less perfect alternative.
- If your sample is dissolved in a solvent: Use a liquid cell and remember to run and subtract a background spectrum of the pure solvent.
- If you have a gaseous sample: You must use a long-path-length gas cell to obtain a spectrum with sufficient signal intensity.
Ultimately, mastering sample preparation is the key to unlocking reliable and informative data from your IR spectrometer.
Summary Table:
| Sample Type | Recommended Method | Key Consideration |
|---|---|---|
| Solid | Pressed KBr Pellet | Grind to fine powder to prevent scattering. |
| Solid | Nujol Mull | Fast, but mineral oil peaks appear in spectrum. |
| Solid (Soluble) | Cast Film | Use a volatile solvent; let evaporate completely. |
| Liquid (Neat) | Thin Film between Salt Plates | Create a capillary film for optimal transmission. |
| Liquid (Solution) | Liquid Cell with Solvent | Subtract solvent background spectrum. |
| Gas | Long-Path-Length Gas Cell | Compensates for low density and weak absorbance. |
Achieve precise and reliable results with every IR analysis. Proper sample preparation is fundamental to data integrity. KINTEK specializes in supplying the high-quality lab equipment and consumables you need for flawless IR spectroscopy, including KBr pellet dies, hydraulic presses, and IR-grade salt plates. Let our experts help you optimize your workflow. Contact our team today to discuss your laboratory's specific needs!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What technical value does a four-column hydraulic press provide? Optimize Your Composite Powder Fabrication Today
- How old is hydraulic technology? From Ancient Waterwheels to Modern Power Systems
- What compression molding is mostly used? For Large, Strong Parts from Thermosets & Composites
- How do you measure the force of a press? Accurately Monitor Tonnage with Direct Load Cell Measurement
- What is the Significance of Using a Laboratory Hydraulic Press for Green Compact Pressing? Optimize CNT Composites.
- How much does a hydraulic press weight? From 20kg Benchtop to Multi-Ton Industrial Giants
- Why is high-precision hydraulic pressing required for oxygen carrier pellets? Ensure Experimental Validity.
- What is the amount of sample required when making a KBr pellet? Achieve Perfect IR Spectra with the 100:1 Ratio



















