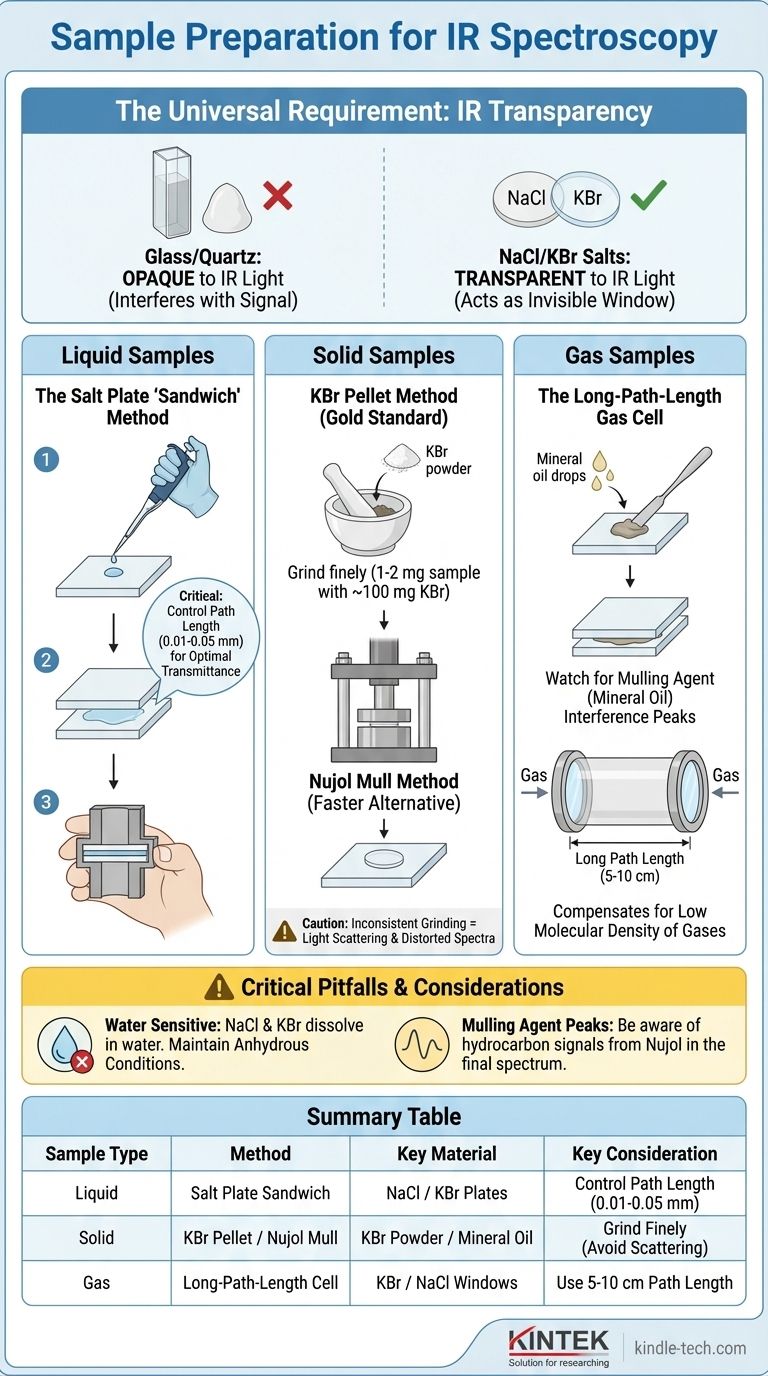
The method for preparing a sample for IR spectroscopy depends entirely on its physical state—whether it is a solid, liquid, or gas. The universal requirement is that the sample must be held in a material that is transparent to infrared radiation, which is why materials like sodium chloride (NaCl) and potassium bromide (KBr) salt plates are used instead of glass or quartz.
The core challenge of IR sample preparation is to present a thin, uniform layer of your material to the spectrometer's beam without introducing any interfering signals. The chosen technique must overcome the fact that common materials like glass are opaque to infrared light, necessitating the use of specialized, often water-sensitive, salt optics.

The Fundamental Principle: IR Transparency
Before examining specific techniques, it's crucial to understand why this preparation is so unique. The entire process is dictated by the need for IR transparency.
Why Standard Labware Fails
Common laboratory materials like glass or quartz strongly absorb infrared radiation in the exact range chemists use for analysis. Placing a sample in a glass cuvette would be like trying to take a picture with the lens cap on; the spectrometer would only see the signal from the glass itself, not the sample.
The Role of Alkali Halide Salts
Materials like sodium chloride (NaCl) and potassium bromide (KBr) are transparent to mid-range infrared light. They act as invisible windows, allowing the IR beam to pass through them and interact only with the sample. This is why they are the standard for IR sample cells and plates.
Preparing Liquid Samples
Preparing a liquid is often the most straightforward method. It involves creating a very thin film of the sample for the IR beam to pass through.
The Salt Plate "Sandwich"
The most common technique is to place one or two drops of the pure liquid sample directly onto a polished salt plate. A second salt plate is then carefully placed on top, spreading the liquid into a thin capillary film. The "sandwich" is then placed in a sample holder in the spectrometer.
Controlling Path Length
The thickness of this liquid film, known as the path length, is critical. For most liquids, a path length of 0.01-0.05 mm is ideal to achieve a transmittance of 15-20%. If the film is too thick, it will absorb too much light, leading to flat-topped, useless peaks.
Preparing Solid Samples
Solid samples cannot be analyzed directly; they must be finely dispersed in an IR-transparent medium to avoid scattering the IR beam.
The KBr Pellet Method
This is considered the gold standard for high-quality solid spectra. A small amount of the solid sample (1-2 mg) is ground into an extremely fine powder with about 100 mg of pure, dry KBr powder. The mixture is then compressed under high pressure in a die to form a small, transparent disc or pellet, which can be placed directly in the sample holder.
The Nujol Mull Method
A Nujol mull is a faster alternative to a KBr pellet. The solid sample is ground to a fine paste with a few drops of a mulling agent, typically a mineral oil like Nujol. This paste is then spread as a thin film between two salt plates, just like a liquid sample.
Preparing Gas Samples
Analyzing gases requires a different approach because they are far less dense than liquids or solids and therefore absorb much less IR radiation.
The Long-Path-Length Gas Cell
Gas samples are introduced into a specialized gas cell. This is essentially a tube sealed at both ends with IR-transparent windows (like KBr or NaCl). To compensate for the low concentration of molecules, these cells have a very long path length, typically 5-10 cm, to ensure the IR beam interacts with enough molecules to produce a measurable signal.
Critical Pitfalls and Considerations
Proper technique is essential to avoid damaging equipment and obtaining misleading results.
The Water Problem
Alkali halide salt plates (NaCl, KBr) are highly soluble in water. Any moisture in the sample, solvent, or even from atmospheric humidity can begin to fog or dissolve the plates, rendering them unusable. All samples and reagents must be anhydrous (water-free).
Interference from Mulling Agents
When using the Nujol mull technique, remember that the mulling oil itself is a hydrocarbon and will produce its own C-H stretching and bending peaks in the spectrum. You must be able to distinguish these known oil peaks from your sample's true signals.
Inconsistent Grinding
For solids, insufficient or uneven grinding is a common source of error. Large particles scatter the IR light, causing a sloping baseline and distorted peak shapes in the final spectrum, making interpretation difficult.
Selecting the Right Method for Your Sample
Your choice of preparation technique depends on the nature of your sample and your analytical goal.
- If your primary focus is a pure liquid or a solution in an IR-transparent solvent: Use the direct salt plate sandwich method for its simplicity and speed.
- If your primary focus is a high-quality spectrum of a stable solid: The KBr pellet method provides the cleanest results without interfering peaks from a mulling agent.
- If your primary focus is a quick analysis of a solid that is air-sensitive or reacts with KBr: The Nujol mull is a practical alternative, provided you can account for the oil's peaks.
- If your primary focus is analyzing a gas or gaseous mixture: A long-path-length gas cell is the only appropriate choice.
Mastering these preparation techniques ensures that your IR spectrum is a true and accurate fingerprint of your sample.
Summary Table:
| Sample Type | Preparation Method | Key Material | Key Consideration |
|---|---|---|---|
| Liquid | Salt Plate 'Sandwich' | NaCl or KBr Plates | Control path length (0.01-0.05 mm) |
| Solid | KBr Pellet or Nujol Mull | KBr Powder or Mineral Oil | Grind finely to avoid light scattering |
| Gas | Long-Path-Length Cell | KBr/NaCl Windows | Use 5-10 cm path length for sufficient signal |
Achieve Perfect IR Spectra with KINTEK's Expertise
Are you struggling with sample preparation or inconsistent IR results? KINTEK specializes in providing the high-quality lab equipment and consumables you need for precise IR spectroscopy. From durable KBr pellets and anhydrous salt plates to reliable gas cells, our products are designed to help you obtain clear, accurate spectra every time.
Let our experts help you select the right materials and techniques for your specific samples. Contact us today to discuss your laboratory needs and discover how KINTEK can support your research and analytical goals.
Get in touch with our team now!
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- What is the importance of using a laboratory hydraulic press for R1/3Zr2(PO4)3 samples? Enhance Ion Conductivity
- How do you clean KBr pellets? The Definitive Guide to Flawless FTIR Analysis
- How does the use of a hydraulic press benefit the thermal reduction process of mixed powders? Optimize Reaction Kinetics
- What is the Bernoulli's principle of hydraulic press? It's Actually Pascal's Law That Powers It
- What is the use of KBr in FTIR? A Key Technique for Accurate Solid Sample Analysis
- How do you convert hydraulic pressure to force? Master the Core Formula for Maximum Power
- What are the advantages of hydraulic press over mechanical press? Flexibility, Control & Cost Savings
- Which mechanism is used in a press machine? Harnessing Hydraulic Power for Maximum Force



















