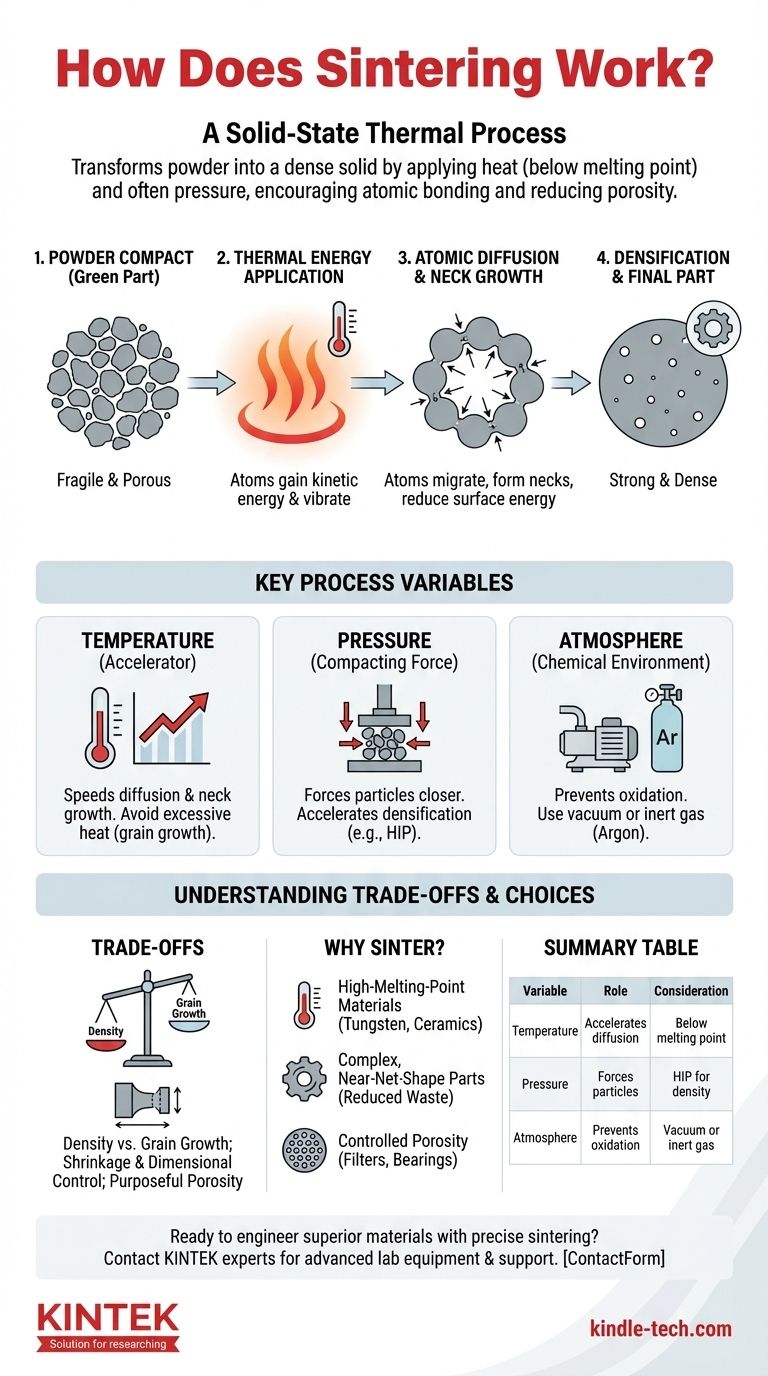
At its core, sintering is a thermal process that transforms a powder-like material into a solid, dense object. It works by applying heat, and often pressure, to a point below the material's melting point. This energy encourages the atoms on the surfaces of individual particles to move and bond with adjacent particles, effectively welding them together on a microscopic scale and reducing the empty space between them.
Sintering should not be confused with melting. It is a solid-state process driven by atomic diffusion, allowing the formation of strong, dense parts from materials that are often difficult or impossible to melt and cast, like advanced ceramics or high-performance metals.

The Fundamental Mechanism: From Powder to Solid
Sintering is fundamentally a process of atomic movement. Understanding this mechanism is key to controlling the properties of the final component.
The Starting Point: A Powder Compact
The process begins with a mass of individual particles, which can be metal, ceramic, or plastic. This mass is often pre-compacted into a desired shape, known as a "green part," which is fragile and porous. The primary goal of sintering is to eliminate these pores.
The Role of Thermal Energy
When the material is heated, the atoms within the solid particles gain kinetic energy. They begin to vibrate more intensely and can even migrate from their fixed positions in the crystal lattice. This atomic mobility is the engine of the entire sintering process.
Atomic Diffusion Across Boundaries
The most critical action occurs at the points where particles touch. Energized atoms diffuse, or move, across the boundaries between adjacent particles. This movement seeks to lower the overall surface energy of the system—a principle similar to how soap bubbles merge to form larger bubbles.
Neck Growth and Densification
As atoms migrate to the contact points, they form small bridges or "necks" between the particles. As the process continues, these necks grow wider, pulling the centers of the particles closer together. This action systematically closes the voids (pores) between the particles, causing the entire part to shrink and become significantly more dense and strong.
Key Process Variables That Control the Outcome
The final properties of a sintered part are not accidental; they are the direct result of carefully controlling three primary variables.
Temperature: The Accelerator
Temperature is the most significant factor influencing the rate of diffusion. Higher temperatures (while still below melting) provide more energy to the atoms, drastically speeding up neck growth and densification. However, excessive temperatures can lead to undesirable grain growth, which may compromise the material's mechanical properties.
Pressure: The Compacting Force
Applying external pressure forces the particles into closer contact, increasing the number of diffusion points and accelerating densification. Processes like Hot Isostatic Pressing (HIP) use both high heat and immense gas pressure to achieve nearly 100% density, which is critical for high-performance applications like turbine blades.
Atmosphere: The Chemical Environment
Sintering rarely happens in open air. The chemical environment is critical because at high temperatures, most materials will readily oxidize. Oxidation forms a layer on the particle surfaces that acts as a barrier, preventing atomic diffusion and bonding. To combat this, sintering is typically performed in a vacuum or in an inert gas atmosphere (like argon), which protects the material.
Understanding the Trade-offs
Sintering is a powerful technique, but it requires balancing competing factors to achieve the desired result.
Density vs. Grain Growth
The primary goal is often to maximize density for strength. However, the high temperatures and long hold times required for full densification can also cause the microscopic crystal grains within the material to grow too large. Overly large grains can make a material more brittle.
Shrinkage and Dimensional Control
Because sintering eliminates porosity, the component will always shrink. This shrinkage can be significant (often 10-20% by volume) and must be precisely calculated and compensated for when designing the initial "green part" mold. Achieving tight dimensional tolerances requires exceptional process control.
Purposeful Porosity
While often seen as a defect to be eliminated, porosity can also be a desired feature. By intentionally arresting the sintering process, engineers can create parts with a controlled network of pores. This is the principle behind self-lubricating bearings (which hold oil) and metallic or ceramic filters.
Making the Right Choice for Your Goal
Deciding to use sintering depends entirely on your material and performance objectives.
- If your primary focus is manufacturing with high-melting-point materials: Sintering is often the only practical method for processing materials like tungsten, molybdenum, and many advanced ceramics that cannot be easily melted and cast.
- If your primary focus is creating complex, near-net-shape parts: Powder metallurgy combined with sintering can dramatically reduce machining waste and subsequent costs compared to starting with a solid block of material.
- If your primary focus is designing for controlled porosity: Sintering provides a unique capability to engineer materials like filters or self-lubricating bearings by intentionally leaving a specific volume of interconnected pores.
By understanding sintering not as melting but as controlled atomic motion, you gain the ability to engineer materials with properties unattainable by other means.
Summary Table:
| Process Variable | Role in Sintering | Key Consideration |
|---|---|---|
| Temperature | Accelerates atomic diffusion for bonding. | Must stay below melting point to avoid grain growth. |
| Pressure | Forces particles closer, speeding densification. | Used in processes like Hot Isostatic Pressing (HIP). |
| Atmosphere | Prevents oxidation (e.g., vacuum, inert gas). | Critical for successful atomic bonding. |
Ready to engineer superior materials with precise sintering?
At KINTEK, we specialize in providing the advanced lab equipment and consumables you need to master thermal processes like sintering. Whether you're working with high-performance metals, advanced ceramics, or developing porous filters, our solutions ensure the temperature, pressure, and atmospheric control required for consistent, high-quality results.
Contact our experts today to discuss how we can support your laboratory's specific sintering and material science goals.
Visual Guide
Related Products
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Spark Plasma Sintering Furnace SPS Furnace
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
People Also Ask
- Why are quartz tubes preferred for chromium powder combustion? Superior Heat Resistance & Optical Clarity
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- What happens when quartz is heated? A Guide to Its Critical Phase Transitions and Uses
- What role does a quartz tube furnace play in hBN synthesis? Optimize Your Chemical Vapor Deposition Results
- What are the primary functions of high-precision tube furnaces in graphene growth? Achieve Defect-Free GS Synthesis



















