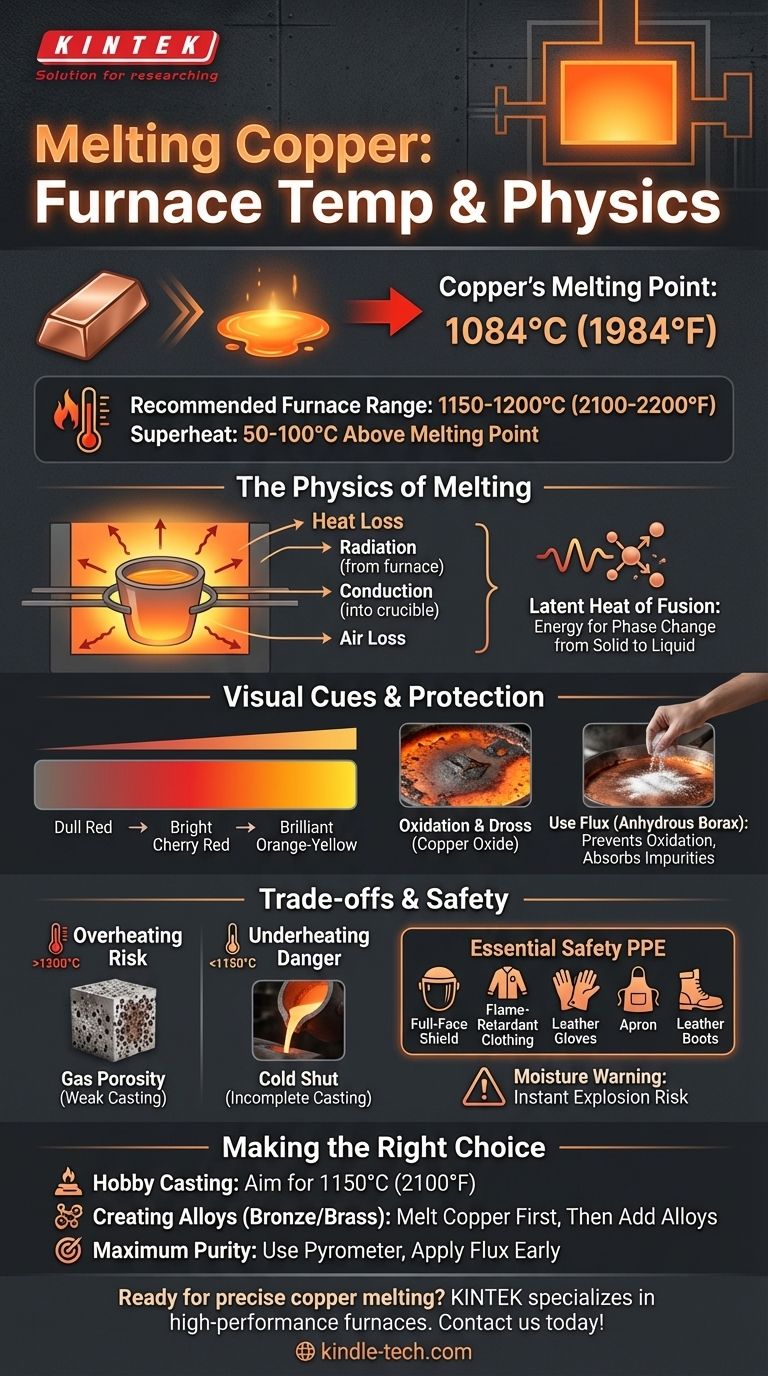
To melt copper, you must heat it to its precise melting point of 1084°C (1984°F). However, to achieve a successful melt and pour, your furnace must be capable of reaching and maintaining a temperature significantly higher than this, typically around 1150-1200°C (2100-2200°F), to account for heat loss and ensure the metal is fully liquid.
The core challenge is not just reaching a specific temperature, but managing heat effectively. While copper melts at a fixed point, successfully casting it requires a hotter furnace to overcome heat loss to the crucible and air, ensuring the metal remains fluid enough for a complete pour.

The Physics of Melting Copper
To melt metal effectively, you need to understand it's not as simple as hitting a target number. You are fighting against the laws of thermodynamics, which dictate where your heat energy goes.
The Specific Melting Point
The temperature of 1084°C (1984°F) is a physical constant for pure copper. At this temperature, the crystalline structure of the solid metal breaks down, and it begins to transition into a liquid state.
The Role of Latent Heat
Simply reaching 1084°C is not enough. You must continue to apply a significant amount of energy, known as the latent heat of fusion, to complete the phase change from solid to liquid. This is why a pile of copper will appear to "stall" at its melting point until it has fully liquefied.
Why Your Furnace Must Be Hotter
Your furnace's heat is constantly escaping. It radiates out from the furnace body, conducts into the crucible holding the copper, and is lost to the surrounding air. To counteract this constant heat loss and provide the necessary latent heat, your furnace must operate at a temperature well above copper's melting point.
A good rule of thumb is to set your furnace to run at least 50-100°C (approx. 100-200°F) hotter than the metal's melting point. This "superheat" ensures the copper melts efficiently and stays hot enough for a successful pour.
From Solid to Liquid: What to Expect
Observing the process is key to understanding the state of your metal. Temperature readings are critical, but visual cues tell a vital part of the story.
Visual Cues of Melting
As the copper heats, it will begin to glow, moving from a dull red to a bright cherry red, and finally to a brilliant orange-yellow as it approaches and surpasses its melting point. The solid pieces will slump, lose their sharp edges, and eventually collapse into a shimmering liquid pool.
The Problem of Oxidation and Dross
Molten copper is highly reactive with oxygen in the air. This reaction forms a dark, crusty layer of copper oxide on the surface known as dross. If mixed into your pour, dross will create weak, porous spots in the final casting.
Using Flux to Protect the Melt
To prevent oxidation, you should use a flux, such as anhydrous borax. Sprinkling a small amount onto the copper as it begins to melt creates a molten glass-like barrier. This layer shields the liquid metal from the air, prevents dross from forming, and absorbs impurities.
Understanding the Trade-offs and Safety
Managing a furnace at these temperatures involves balancing risks. Both overheating and underheating create distinct problems, and safety must always be the highest priority.
The Risk of Overheating
Excessively high temperatures (well above 1200°C / 2200°F) can be detrimental. It increases the amount of gas (like hydrogen and oxygen) that dissolves into the molten copper. This gas will come out of solution as the metal cools, creating tiny bubbles and resulting in a weak, porous casting—a defect known as gas porosity.
The Danger of Underheating
Failing to superheat the copper sufficiently is a common beginner's mistake. If the metal is too close to its melting point, it may begin to solidify in transit from the crucible to the mold. This results in a "cold shut" or an incomplete casting, where the metal freezes before filling the entire mold cavity.
Essential Safety Precautions
Working with molten metal is extremely dangerous. Non-negotiable personal protective equipment (PPE) includes a full-face shield (not just glasses), flame-retardant clothing, leather gloves, an apron, and leather boots. Any moisture—even a drop of sweat—contacting molten metal will instantly flash to steam, causing a violent explosion of liquid metal.
Making the Right Choice for Your Goal
Your target temperature depends on what you intend to accomplish with the molten copper. Adjust your approach based on your specific objective.
- If your primary focus is small-scale hobby casting: Aim for a pour temperature of around 1150°C (2100°F). This provides enough fluidity to capture detail in molds without excessive risk of gas porosity.
- If your primary focus is creating alloys like bronze or brass: Melt the copper completely first. Only then should you add lower-melting-point metals like tin or zinc to prevent them from vaporizing and burning off before the copper is liquid.
- If your primary focus is maximum purity and control: Use a pyrometer to precisely measure the metal's temperature, not just the furnace air. Apply a light layer of flux as soon as the metal becomes liquid to minimize oxidation.
Mastering the process is about understanding the properties of the material, not just memorizing a number.
Summary Table:
| Key Temperature | Purpose |
|---|---|
| 1084°C (1984°F) | Copper's Melting Point |
| 1150-1200°C (2100-2200°F) | Recommended Furnace Operating Range |
| 50-100°C Above Melting Point | Superheat for Efficient Pouring |
Ready to achieve precise, safe, and efficient copper melting? KINTEK specializes in high-performance lab furnaces and consumables designed for metallurgy and casting applications. Our equipment ensures accurate temperature control and durability, helping you avoid defects like gas porosity and cold shuts. Contact our experts today to find the perfect furnace for your laboratory needs!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- How does a quartz tube facilitate fractional condensation in a horizontal tube vacuum gasification furnace? Expert Guide
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?
- How does a high-temperature tube furnace facilitate the phase transformation of alumina products? Master Thermal Control
- What is a tubular furnace used for? Precision Heating for Material Synthesis & Analysis
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance



















