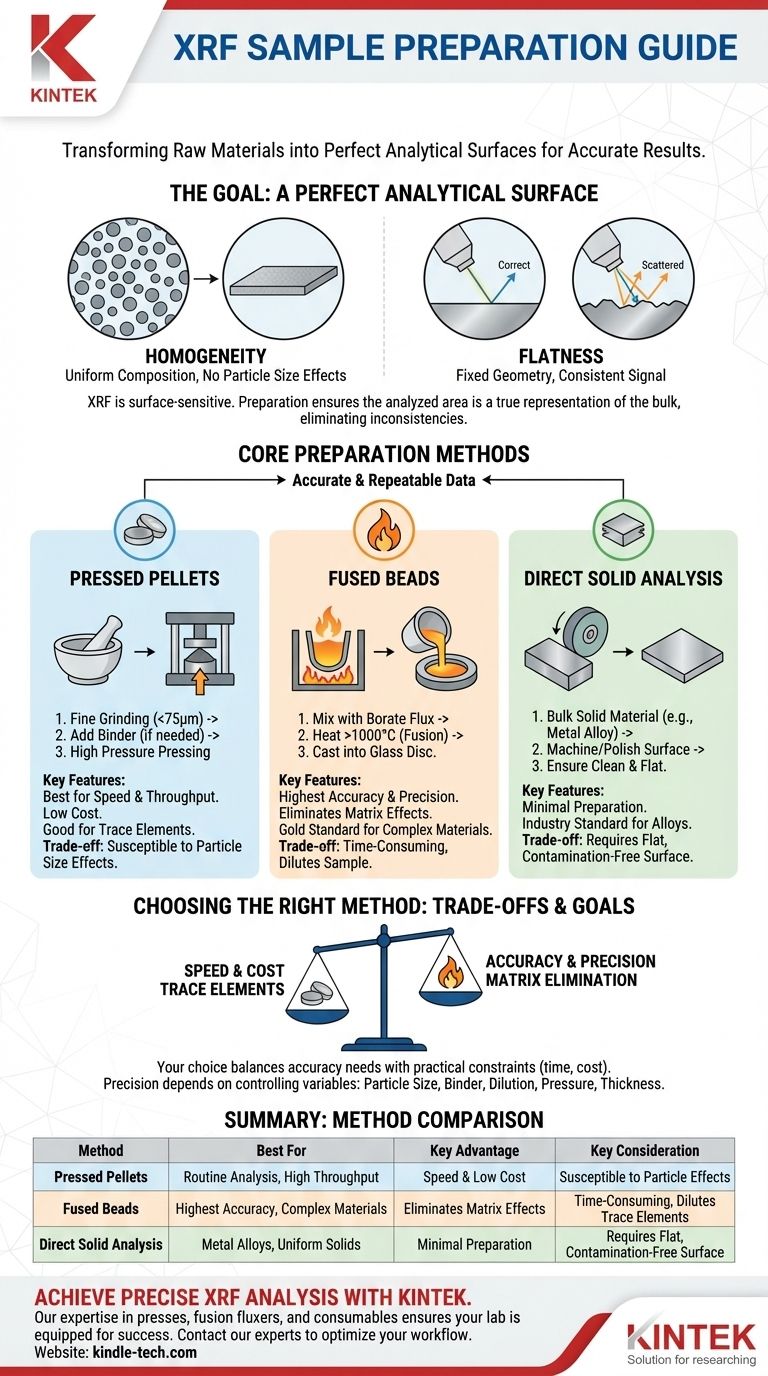
At its core, preparing a sample for X-ray fluorescence (XRF) analysis involves a series of physical steps—typically crushing, grinding, and then pressing or fusing the material. The universal goal is to transform the raw material into a perfectly homogeneous sample with a flat, clean surface for the instrument to analyze. This meticulous preparation is what ensures the final data is both accurate and repeatable.
The fundamental challenge in XRF sample preparation is not just about handling the material, but about eliminating physical inconsistencies. Your choice of method, from a simple polished surface to a complex fused bead, is a deliberate trade-off between speed, cost, and the level of analytical precision your goal requires.

The Goal of Preparation: A Perfect Analytical Surface
XRF is a surface-sensitive technique. The instrument analyzes a specific area, and it assumes that this small area is a perfect representation of your entire sample. Any physical variation can distort the results.
Why Homogeneity is Non-Negotiable
An XRF instrument reads fluorescent X-rays emitted from the sample's surface. If that surface has inconsistent particle sizes or mineral composition, the results will be skewed and unreliable.
Proper preparation, like fine grinding, ensures that the analyzed area is a true average of the bulk material, eliminating these "particle size effects."
The Critical Role of a Flat Surface
The geometry between the X-ray source, the sample, and the detector is fixed. Any surface roughness, voids, or unevenness will scatter the X-rays unpredictably, weakening the signal and producing inaccurate data.
The final prepared sample, whether a pressed pellet or a solid block, must be flawlessly flat to ensure consistent and reliable measurements.
Core Preparation Methods
The method you choose depends heavily on the sample type and the required data quality. Solids are typically prepared using one of three main approaches.
Method 1: Pressed Pellets
This is the most common method due to its speed and low cost. The process involves grinding the sample into a very fine powder, typically smaller than 75 micrometers.
This powder is then poured into a die and pressed under high pressure (several tons) to form a dense, stable pellet. If the powder does not bind well on its own, a small amount of wax binder is added to help it cohere.
Method 2: Fused Beads
For the highest accuracy, especially with complex geological materials, fusion is the gold standard. This method eliminates virtually all particle size and mineralogical effects.
The sample is mixed with a lithium borate flux and heated in a crucible to over 1000°C until it melts completely. The molten glass is then cast into a mold to form a perfectly homogeneous glass disc, known as a fused bead.
Method 3: Direct Analysis of Bulk Solids
For solid, uniform materials like metal alloys or polymers, preparation can be much simpler. The goal is to create a clean, representative, and flat surface on the bulk material itself.
This is typically achieved by machining, lathing, or grinding the surface. It is critical to use dedicated tools for different metal types to prevent cross-contamination, which can introduce false elements into the analysis.
Understanding the Trade-offs
No single method is universally superior. Your choice must balance the need for accuracy against practical constraints like time and cost.
Pressed Pellets: Speed vs. Physical Effects
The primary advantage of pressed pellets is speed and high throughput. They also undergo less dilution, which is better for measuring trace elements.
However, they are more susceptible to errors from particle size variations and mineralogical differences within the sample. A poorly pressed pellet can also be fragile.
Fused Beads: Accuracy vs. Complexity and Dilution
Fusion creates a nearly perfect sample, offering the highest level of accuracy and precision by dissolving the entire sample matrix into glass.
The main downsides are the time and equipment required. The process also dilutes the sample in flux, which can lower the signal for trace elements below the instrument's detection limit.
Key Variables to Control
Regardless of the method, precision depends on controlling key factors. These include the final particle size, the type and amount of binder, the sample-to-flux dilution ratio, the pressure applied to a pellet, and the final sample thickness.
Choosing the Right Method for Your Goal
Your analytical objective should guide your preparation strategy. There is no one-size-fits-all answer, only the best approach for a specific task.
- If your primary focus is speed and routine process control: Pressed pellets are the most efficient choice for analyzing large batches of consistent material.
- If your primary focus is the highest possible accuracy and certifying materials: Fused beads provide the most reliable and repeatable results by completely eliminating physical matrix effects.
- If your primary focus is analyzing solid metal alloys: Direct surface preparation via polishing or lathing is the industry standard, assuming you can create a clean and flat surface.
Ultimately, methodical and consistent sample preparation is the foundation upon which all trustworthy XRF data is built.
Summary Table:
| Preparation Method | Best For | Key Advantage | Key Consideration |
|---|---|---|---|
| Pressed Pellets | Routine analysis, high throughput | Speed and low cost | Susceptible to particle size effects |
| Fused Beads | Highest accuracy, complex materials | Eliminates matrix effects | Time-consuming; dilutes trace elements |
| Direct Solid Analysis | Metal alloys, uniform solids | Minimal preparation | Requires a flat, contamination-free surface |
Achieve precise and reliable XRF analysis with KINTEK.
Your sample preparation method is the foundation of your data's accuracy. Whether you require the speed of pressed pellets or the ultimate precision of fused beads, KINTEK's expertise in lab equipment and consumables ensures your laboratory is equipped for success.
We provide the reliable presses, fusion fluxers, and high-quality consumables you need to prepare perfect samples, time after time. Let our specialists help you select the ideal preparation solution for your specific materials and analytical goals.
Contact our experts today to optimize your XRF workflow and ensure your results are always trustworthy.
Visual Guide
Related Products
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
People Also Ask
- Why is a hydraulic pellet press used for FTIR? Transform Nanofillers into Clear Data
- How is a laboratory hydraulic press used for LLZTO pellets? Achieve 93% Density in Solid-State Battery Research
- How does a laboratory manual hydraulic press facilitate the FT-IR characterization of catalysts? Master Sample Prep.
- How do laboratory hydraulic presses facilitate biomass pelletization? Optimize Biofuel Density and Prevent Slagging
- What is the function of a laboratory hydraulic press for Li10GeP2S12 pellets? Optimize Solid-State Battery Performance



















