At its core, Chemical Vapor Deposition (CVD) creates graphene by flowing a carbon-containing gas over a heated metal substrate inside a vacuum chamber. The high temperature causes the gas to decompose, depositing carbon atoms onto the metal surface, which acts as a catalyst. These atoms then self-assemble into the characteristic hexagonal lattice of a single graphene sheet.
While many methods can produce graphene, they often struggle with scale and quality. Chemical Vapor Deposition is the most promising industrial-scale technique because it allows for the growth of large, continuous, and high-quality single-layer graphene sheets.

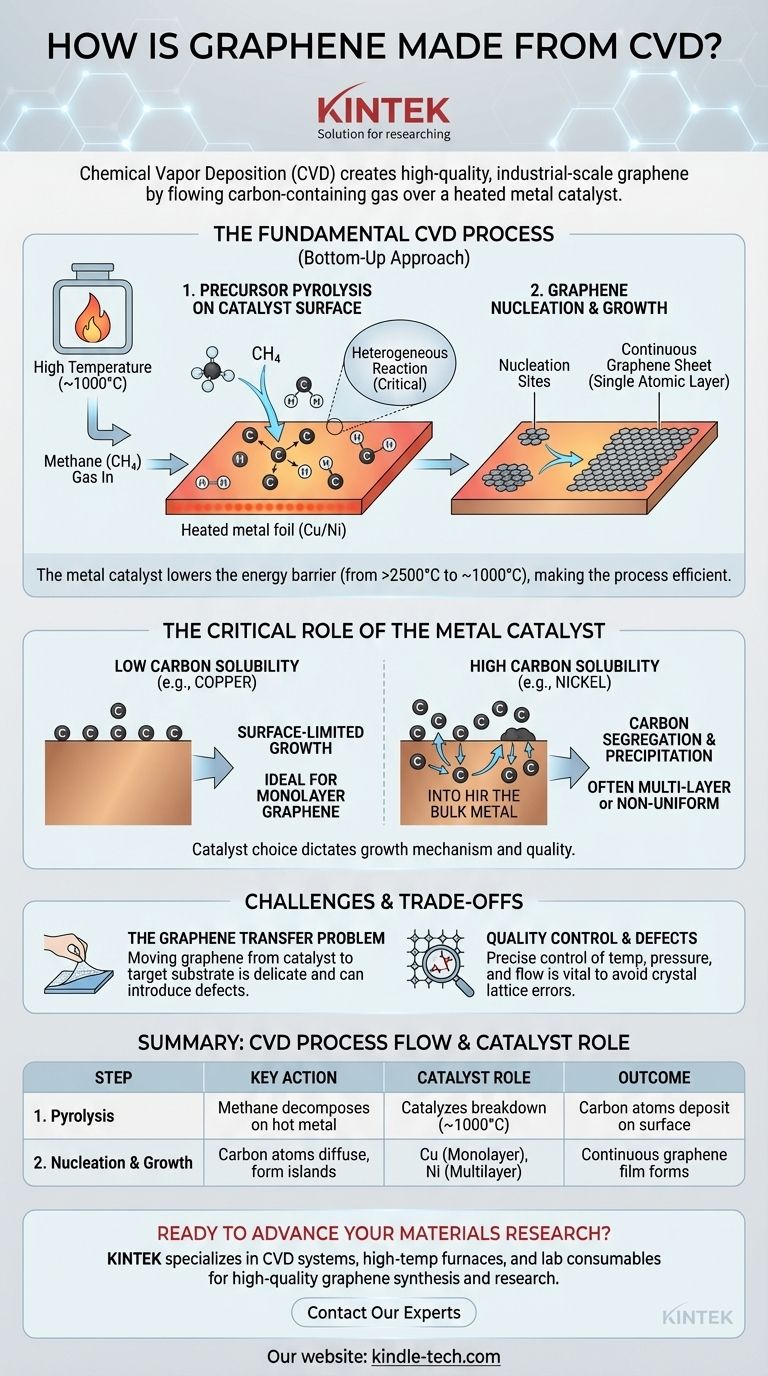
The Fundamental CVD Process: A Two-Step Breakdown
The entire CVD process for graphene synthesis is a "bottom-up" approach, meaning the material is built atom by atom. This process can be distilled into two essential steps that occur at high temperatures (around 1000 °C).
Step 1: Precursor Pyrolysis on the Catalyst Surface
First, a carbon precursor gas, most commonly methane (CH₄), is introduced into the reactor chamber.
The gas flows over a heated metal substrate, typically a thin foil of copper (Cu) or nickel (Ni). The intense heat causes the precursor gas molecules to break apart, or pyrolyze, into reactive carbon atoms and other species.
It is critical that this decomposition happens on the hot metal surface (heterogeneous reaction). If it occurs in the gas phase away from the substrate, the carbon atoms can clump together to form soot, which degrades the quality of the final graphene film.
Step 2: Graphene Nucleation and Growth
Once free carbon atoms are available on the metal surface, they begin to diffuse and arrange themselves.
These mobile atoms eventually form small, stable clusters that act as nucleation sites. From these points, the graphene crystal begins to grow as more carbon atoms attach to the edges of the initial islands.
Over time, these individual islands expand and merge, ultimately forming a continuous, single atomic layer of graphene that covers the entire surface of the metal substrate.
The Critical Role of the Metal Catalyst
The metal substrate is not just a passive surface; it is an active catalyst that is fundamental to the entire process. Without it, the reaction would be impractical.
Lowering the Energy Barrier
The primary role of the catalyst is to dramatically lower the activation energy required for the two steps.
Without a catalyst, forming the graphitic structure would require temperatures exceeding 2500°C. The metal substrate enables the process to occur efficiently at a much more manageable ~1000°C, saving enormous amounts of energy and simplifying reactor design.
Dictating the Growth Mechanism
The choice of metal catalyst also determines how the graphene forms, which directly impacts the final quality and number of layers. This is governed by the metal's carbon solubility.
For a metal with low carbon solubility, like copper (Cu), the process is surface-limited. Carbon atoms cannot dissolve into the bulk copper, so they remain on the surface and form a single layer. Once the surface is covered, growth effectively stops, making copper the ideal catalyst for producing high-quality monolayer graphene.
For a metal with high carbon solubility, like nickel (Ni), the mechanism is different. At high temperatures, carbon atoms dissolve and diffuse into the bulk metal. As the substrate is cooled, the carbon's solubility decreases, causing it to precipitate, or segregate, back to the surface to form graphene. This process is harder to control and often results in multi-layer or non-uniform graphene.
Understanding the Trade-offs and Challenges
While CVD is a powerful technique, it is not without its complexities and challenges that require careful management.
The Graphene Transfer Problem
CVD graphene is grown on a metal catalyst, but it is almost always used on a different substrate, like silicon or a flexible polymer. This requires a difficult transfer process.
The graphene film must be carefully lifted off the metal foil and moved to the target substrate, a delicate procedure that can introduce wrinkles, tears, and contamination, compromising the material's exceptional properties.
Quality Control and Defects
The final quality of the graphene film is extremely sensitive to process conditions.
Variables like temperature, gas pressure, and flow rates must be precisely controlled. Improper conditions can lead to the formation of defects in the crystal lattice or the growth of undesirable multi-layer patches, even on a copper substrate.
Making the Right Choice for Your Goal
The optimal CVD approach depends entirely on the specific type of graphene you intend to produce. By understanding the core principles, you can tailor the process to your needs.
- If your primary focus is large-area, high-quality monolayer graphene: Use a low carbon solubility catalyst like copper foil, as its surface-limited growth mechanism is self-regulating.
- If your primary focus is exploring multi-layer graphene: A high carbon solubility catalyst like nickel can be used, but you must precisely control the cooling rate to manage carbon segregation.
- If your primary focus is process optimization and research: Concentrate on the interplay between temperature and precursor gas flow to control the density of nucleation sites and the final grain size of the graphene film.
Ultimately, mastering the CVD process is the key to transitioning graphene from a laboratory wonder to an industrial-scale material.
Summary Table:
| CVD Process Step | Key Action | Catalyst Role | Outcome |
|---|---|---|---|
| 1. Precursor Pyrolysis | Methane gas decomposes on hot metal surface (e.g., Cu, Ni) | Catalyzes gas breakdown at ~1000°C (vs. 2500°C without catalyst) | Carbon atoms deposit on catalyst surface |
| 2. Nucleation & Growth | Carbon atoms diffuse and form hexagonal lattice islands | Cu (low solubility) enables monolayer growth; Ni (high solubility) enables multilayer growth | Continuous graphene film forms |
Ready to advance your materials research with precision lab equipment? KINTEK specializes in providing the CVD systems, high-temperature furnaces, and essential lab consumables you need to synthesize and study high-quality graphene. Our expertise supports laboratories in optimizing growth parameters and achieving reliable, reproducible results. Contact our experts today to discuss how our solutions can accelerate your graphene innovation!
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
People Also Ask
- What are the precursors used in CVD synthesis of graphene? Key Choices for High-Quality Growth
- What are sputtering systems? A Guide to High-Quality Thin Film Deposition
- Can you tell the difference between CVD and Hpht diamonds? A Guide to Choosing the Right Lab-Grown Diamond
- What are the techniques of deposition in semiconductors? Mastering CVD, PVD, and ALD for Chip Fabrication
- What is the temperature of chemical Vapour deposition? A Guide to High & Low-Temp CVD Processes
- What are the characteristics of a hot-wall CVD system? Key Benefits & Architectural Insights
- What is physical vapor deposition used for? Enhancing Durability, Function, and Appearance
- What is an example of MOCVD? The Key Process for High-Performance LEDs & Lasers



















