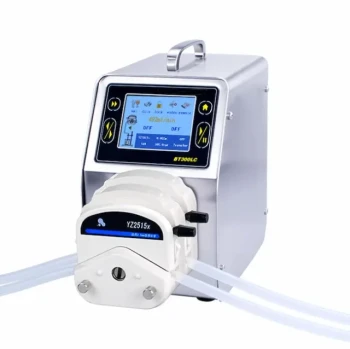
In any rigorous scientific experiment, temperature is controlled using a closed-loop feedback system. This system consists of a sensor to measure the current temperature, a controller to compare that measurement to the desired setpoint, and an actuator (a heater or cooler) that actively adjusts the temperature. Common methods for implementing this include water baths, incubators, solid-state Peltier devices, and cryogenic systems, each chosen based on the specific needs of the experiment.
The core challenge of temperature control is not simply reaching a target number, but achieving the necessary stability, uniformity, and responsiveness for your specific application. The method you choose is a direct trade-off between these critical performance factors.

The Core Principle: The Feedback Loop
At its heart, all modern temperature control operates on a simple but powerful concept: a feedback loop. This loop has three essential components working in constant concert.
The Sensor: Measuring Reality
The sensor is the "eyes" of the system. It continuously measures the actual temperature of your sample or its immediate environment.
Common sensor types include thermocouples, thermistors, and resistance temperature detectors (RTDs). The choice of sensor depends on the required temperature range, accuracy, and cost.
The Controller: The Brain of the Operation
The controller is the decision-making unit. It takes the reading from the sensor and compares it to the setpoint temperature you have programmed.
Based on the difference (the "error"), the controller calculates how much heating or cooling is needed. Simple controllers just turn on or off, but advanced PID (Proportional-Integral-Derivative) controllers make sophisticated adjustments to prevent overshooting the target and to maintain exceptional stability.
The Actuator: Taking Action
The actuator is the component that physically does the work of changing the temperature. It receives commands from the controller and applies energy to or removes it from the system.
Common actuators include resistive heating elements (like in an oven), thermoelectric coolers (Peltier devices) that can both heat and cool, and compressors or cryogen valves for more powerful cooling.
Common Methods for Temperature Control
The feedback loop principle is applied through various types of equipment, each suited for different experimental contexts.
Fluid Baths (Water or Oil)
This method involves immersing the experiment in a tank of stirred liquid. The large thermal mass of the fluid acts as a powerful buffer against temperature fluctuations.
They provide excellent temperature stability and uniformity around the sample. However, their response time to setpoint changes is slow, and their temperature range is limited by the fluid's properties (e.g., water boils at 100°C).
Incubators and Ovens (Forced Air)
These enclosed chambers use a heating element and a fan to circulate warm air around the samples. This is a common method for cell cultures and microbiology.
Their strength is handling large volumes or numerous samples simultaneously. The primary weakness is the potential for temperature gradients, where some areas inside the chamber are hotter or colder than others.
Peltier Devices (Thermoelectric Control)
A Peltier device is a solid-state heat pump. Applying a DC current moves heat from one side of the device to the other. Reversing the current reverses the direction of heat flow.
This allows for incredibly fast and precise heating and cooling in a small package with no moving parts. They are ideal for applications like PCR thermal cyclers or controlling the temperature of a single microscope slide, but they are inefficient for cooling large volumes.
Cryostats and Cryocoolers (Cryogenic Control)
For experiments below ambient temperature, specialized systems are required. These often use a liquid cryogen like liquid nitrogen (LN2) or a mechanical refrigeration cycle.
These are essential for materials science, low-temperature physics, and flash-freezing biological samples. They are complex and expensive but are the only way to achieve stable and controlled cryogenic temperatures.
Understanding the Trade-offs
No single method is perfect for every situation. Choosing the right one requires understanding the inherent compromises.
Stability vs. Speed
A large, insulated water bath offers exceptional stability but takes a very long time to change temperature. A Peltier device can change temperature in seconds but requires a sophisticated PID controller to hold that temperature with high stability.
Uniformity vs. Simplicity
Placing a beaker on a simple hot plate is easy, but it creates a massive temperature gradient through the liquid. A stirred fluid bath is a more complex setup but ensures the entire sample is at a uniform temperature, which is critical for reaction kinetics.
Cost vs. Precision
A basic laboratory oven might cost a few hundred dollars but only hold a temperature to within a few degrees. A high-precision differential scanning calorimeter, which relies on exquisitely controlled temperature ramps, can cost tens of thousands of dollars. The required precision directly dictates the cost and complexity.
The Problem of Overshoot
A simple on/off controller (like a home thermostat) will always cause temperature overshoot and undershoot. The heater turns on until the setpoint is reached, but residual heat continues to raise the temperature past the target. For science, this oscillation is often unacceptable, which is why PID controllers are the industry standard for precision.
Choosing the Right Method for Your Experiment
The ideal method depends entirely on the demands of your sample and your scientific goal.
- If your primary focus is long-term cell culture: An incubator with good air circulation and humidity control provides the stable environment needed for biological growth.
- If your primary focus is precise enzyme kinetics: A circulating water bath or a Peltier-controlled cuvette holder ensures the reaction rate is measured at a highly stable and uniform temperature.
- If your primary focus is rapid thermal cycling (like PCR): A system built on Peltier elements is essential for its ability to heat and cool quickly and accurately between specific temperatures.
- If your primary focus is studying material properties at low temperatures: You have no choice but to use a cryostat or a dedicated cryocooler system designed for that range.
Ultimately, understanding these control principles empowers you to select a tool that ensures your experimental results are both accurate and repeatable.
Summary Table:
| Control Method | Best For | Key Strengths | Key Limitations |
|---|---|---|---|
| Fluid Baths (Water/Oil) | Stable, uniform heating/cooling | Excellent stability & uniformity | Slow response, limited temperature range |
| Incubators/Ovens (Forced Air) | Cell culture, large sample volumes | Handles large volumes well | Potential for temperature gradients |
| Peltier Devices | Rapid thermal cycling (e.g., PCR) | Fast, precise heating & cooling | Inefficient for large volumes |
| Cryostats/Cryocoolers | Cryogenic temperature studies | Stable ultra-low temperatures | Complex and expensive |
Achieve Unmatched Experimental Precision with KINTEK
Whether your research requires the rapid thermal cycling of a PCR machine, the stable environment of an incubator, or the cryogenic control of a materials science study, the right temperature control system is critical for valid, repeatable results.
At KINTEK, we specialize in providing high-performance lab equipment tailored to your specific experimental needs. Our expertise ensures you get the precision, stability, and responsiveness your work demands.
Let us help you eliminate temperature-related variables from your research. Contact our experts today to find the perfect solution for your laboratory.
Visual Guide
Related Products
- Shaking Incubators for Diverse Laboratory Applications
- Infrared Heating Quantitative Flat Plate Press Mold
- Manual Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Custom PTFE Teflon Parts Manufacturer PTFE Beaker and Lids
People Also Ask
- What is the purpose of a constant temperature incubator shaker? Master Quercetin Adsorption on Nanocomposites
- What critical reaction conditions does a shaking incubator provide? Optimize Cassava Cellulose Enzymatic Hydrolysis
- What function does a constant temperature shaker perform during adsorption performance tests? Ensure Data Accuracy
- Why is a constant temperature shaking incubator necessary for antibacterial experiments? Ensure Valid Results
- Why is a constant temperature shaker required during the impregnation of manganese salts onto activated carbon?