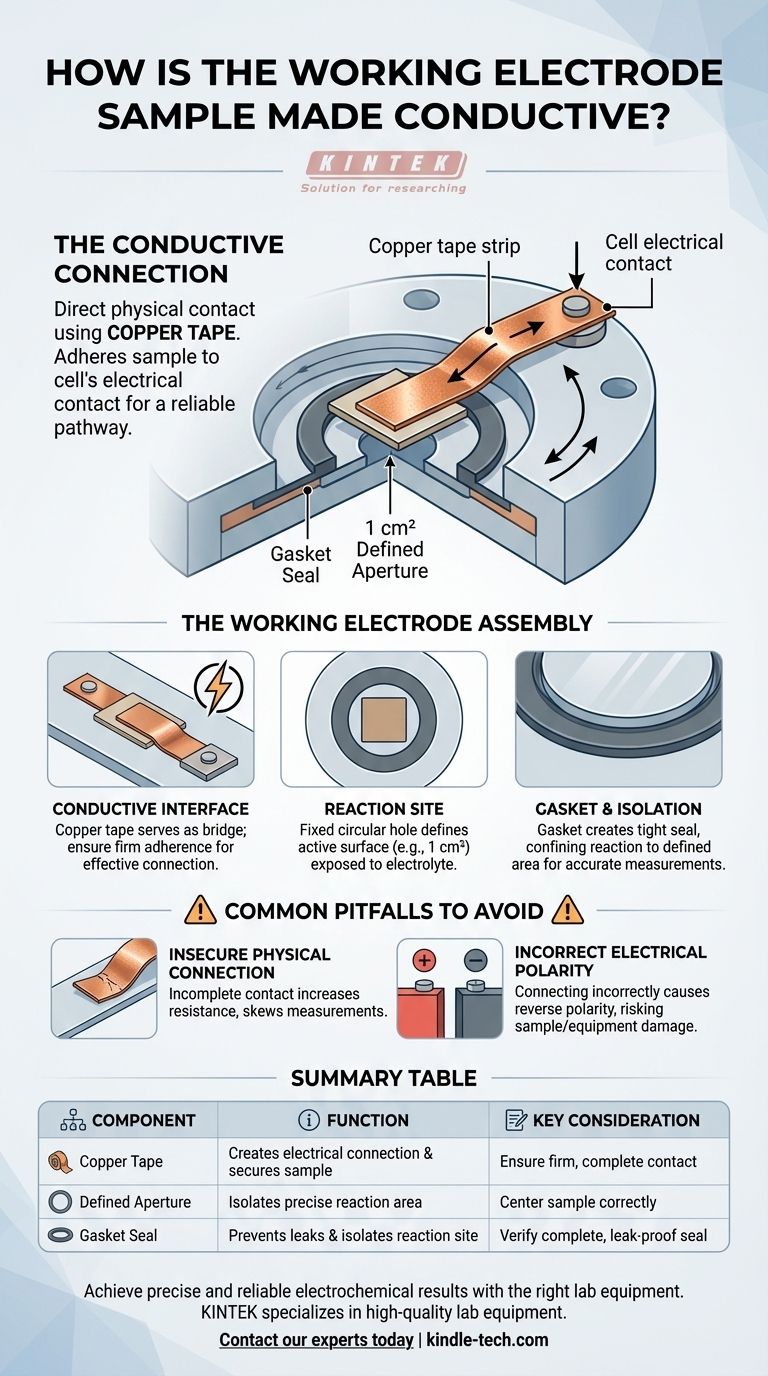
To establish a conductive connection for the working electrode's sample, the method employed is direct physical contact using copper tape. This tape is used to adhere the sample piece to the cell's electrical contact, creating a reliable pathway for electrical current to the material being studied.
The core principle is straightforward: conductivity is achieved by physically securing the sample with copper tape. However, the integrity of the experiment equally depends on how this sample is integrated into the cell's physical design, which uses a sealed gasket and a defined aperture to isolate the precise electrochemical reaction area.

The Mechanics of the Working Electrode Assembly
A successful electrochemical measurement relies on understanding how each component contributes to the final setup. The working electrode assembly is more than just a single connection; it's a small, integrated system.
The Conductive Interface: Copper Tape
The primary method for ensuring conductivity is the use of copper tape. This material serves as the bridge between the external power supply connection and the sample itself.
The tape is used to adhere to the sample, physically holding it in place while simultaneously creating an electrical pathway. The effectiveness of this connection is critical for accurate results.
The Reaction Site: A Defined Area
The cell design features a fixed circular hole at its base, which has a standard area of 1 square centimeter. This aperture is what defines the active surface of your working electrode.
Only the portion of the sample exposed through this hole will come into contact with the electrolyte and participate in the electrochemical reaction. The size of this hole can also be customized.
The Seal: Gasket and Isolation
To prevent leaks and ensure that the reaction is confined only to the defined area, a gasket is used to create a tight seal.
This isolation is crucial for calculating metrics like current density, as it ensures the reactive surface area is known and constant.
Common Pitfalls to Avoid
Proper assembly is critical for both safety and data integrity. A few key details require close attention during setup.
Insecure Physical Connection
An incomplete or loose connection between the copper tape and the sample can introduce unwanted electrical resistance.
This can skew measurements and lead to erroneous data. The tape must be applied firmly to ensure a solid, continuous conductive path.
Incorrect Electrical Polarity
The power supply must be connected with the correct polarity. Connecting the positive and negative terminals incorrectly can cause reverse polarity.
This error can lead to unintended chemical reactions, damage to the sample, or harm to the electrode itself. Always double-check your connections before applying power.
Making the Right Choice for Your Goal
Your experimental objective will determine which aspect of the setup requires the most attention.
- If your primary focus is accurate measurement: Ensure the copper tape makes a complete and firm connection to the sample to minimize any contact resistance.
- If your primary focus is reproducibility: Confirm that your sample is perfectly centered over the 1 cm² aperture and that the gasket provides a complete, leak-proof seal every single time.
- If your primary focus is equipment safety: Always double-check the positive and negative terminal connections to your power supply before beginning to prevent reverse polarity.
Understanding these simple mechanical and electrical principles is the key to achieving reliable and meaningful experimental results.
Summary Table:
| Component | Function | Key Consideration |
|---|---|---|
| Copper Tape | Creates electrical connection & secures sample | Ensure firm, complete contact to minimize resistance |
| Defined Aperture | Isolates the precise reaction area (e.g., 1 cm²) | Center sample correctly for accurate surface area |
| Gasket Seal | Prevents leaks and isolates the reaction site | Verify a complete, leak-proof seal every time |
Achieve precise and reliable electrochemical results with the right lab equipment.
Proper sample preparation and cell assembly are fundamental to your experiment's success. KINTEK specializes in high-quality lab equipment and consumables, including reliable components for your electrochemical setups. Our expertise ensures you have the right tools for accurate measurement, reproducibility, and equipment safety.
Let us help you optimize your lab's capabilities. Contact our experts today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Nickel Aluminum Tabs for Soft Pack Lithium Batteries
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- FS Electrochemical Hydrogen Fuel Cells for Diverse Applications
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What does a layered film mean? Unpacking the Depths of Cinematic Storytelling
- What is the correct shutdown procedure after an experiment? A Step-by-Step Guide to Safe Deactivation
- What procedures should be followed after using nickel or copper foam? A Guide to Reliable Reuse and Performance
- What is the purpose of laminating? Protect and Enhance Your Documents for Long-Term Use
- What are the examples of electrode materials? From Platinum to Graphite for Your Application
















