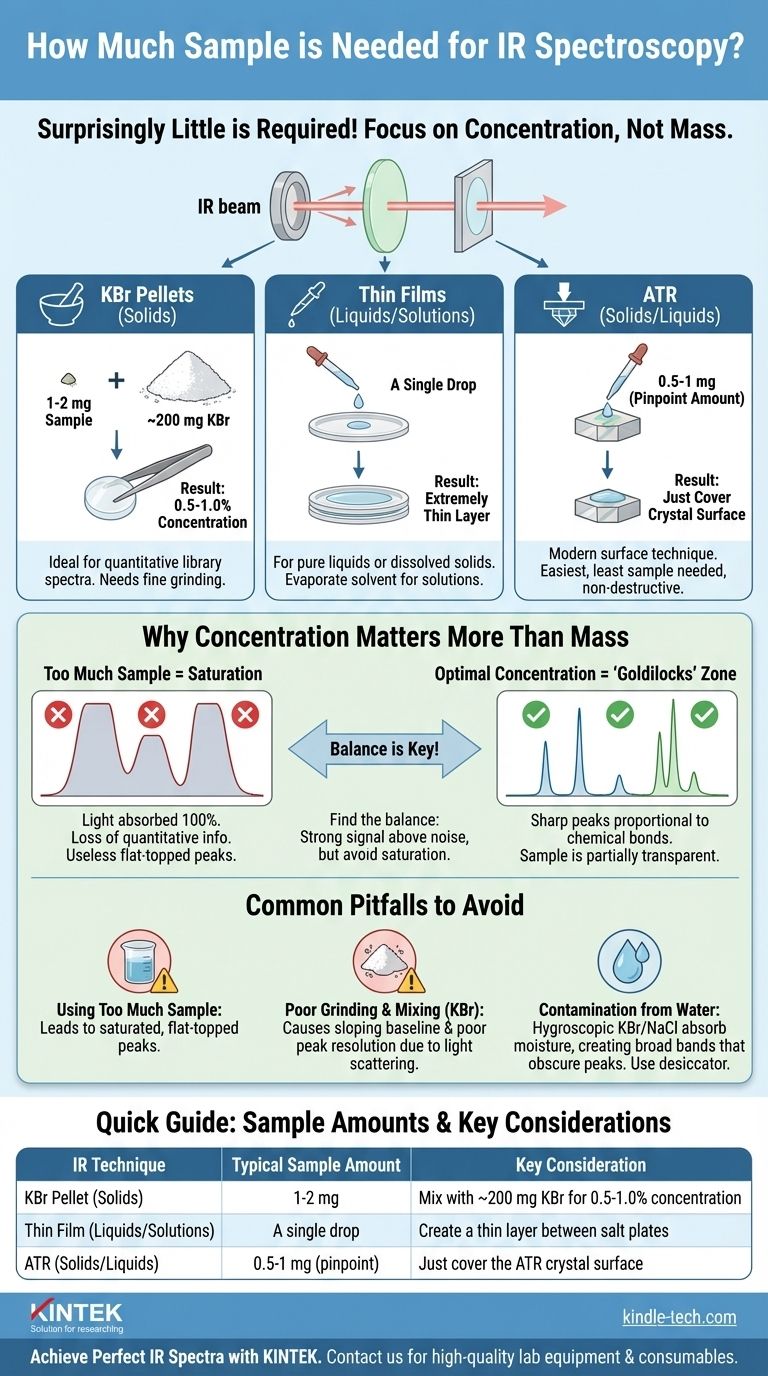
For most infrared (IR) spectroscopy techniques, you need surprisingly little sample. For solid samples prepared using a KBr pellet, typically only 1-2 milligrams (mg) are required. For pure liquids or solutions analyzed as a thin film, a single drop is often sufficient. The exact amount depends entirely on the sample preparation method you choose.
The critical factor in IR spectroscopy is not the total mass of your sample, but its concentration in the path of the IR beam. The goal is to use just enough material to absorb light without oversaturating the detector, which results in useless, flat-topped peaks.

Why Sample Concentration Matters More Than Mass
The fundamental goal is to get a spectrum where the peaks are sharp and proportional to the amount of a specific chemical bond. This is only possible when the sample is partially transparent to the infrared radiation.
The Principle of IR Transparency
For the spectrometer's detector to measure how much light was absorbed at each frequency, some of that light must pass through the sample to reach it.
If the sample is too concentrated or too thick, it will absorb nearly 100% of the IR light in certain regions. The detector simply sees darkness, and you cannot get meaningful information.
The Problem of Signal Saturation
When a sample is too concentrated, the resulting peaks in the spectrum appear broad and flat-topped. This is called saturation or a cutoff peak.
These saturated peaks are problematic because you lose all quantitative information. You cannot tell the difference between a sample that is 2x too concentrated and one that is 10x too concentrated—both will simply max out the detector's reading.
Finding the "Goldilocks" Concentration
Proper sample preparation is about finding the right balance. You need enough sample to produce a strong signal well above the background noise, but not so much that you saturate the strongest absorption bands.
Sample Requirements for Common IR Techniques
The amount of sample needed varies significantly based on the technique used. Each method is designed to control the sample's effective concentration.
For KBr Pellets (Solids)
This is a classic transmission method. You mix a small amount of your solid sample with a large amount of IR-transparent salt, typically potassium bromide (KBr).
The standard ratio is about 1-2 mg of sample finely ground with ~200 mg of dry KBr. This creates a final concentration of 0.5% to 1.0%, ensuring the resulting pellet is sufficiently transparent.
For Thin Films (Liquids/Solutions)
This method is used for pure liquids or solids dissolved in a volatile solvent.
A single drop of the liquid is placed between two IR-transparent salt plates (like NaCl or KBr). The plates are gently pressed together to create an extremely thin film. For solutions, the drop is placed on a single plate and the solvent is evaporated, leaving the thin film of the solid behind.
For Attenuated Total Reflectance (ATR)
ATR is a modern surface technique that requires the least amount of sample and is often the easiest method.
You only need enough solid or liquid sample to completely cover the small ATR crystal surface. This can be as little as 0.5 to 1 mg, and often just a pinpoint amount is sufficient. The IR beam only penetrates a few micrometers into the sample, making concentration less of a concern.
Common Pitfalls to Avoid
Getting a good spectrum is less about the exact mass and more about avoiding common preparation errors.
Using Too Much Sample
This is the most common mistake. It directly leads to the saturated, flat-topped peaks discussed earlier, rendering your spectrum unusable for analysis. Always start with less sample than you think you need.
Poor Grinding and Mixing (KBr Pellets)
If the sample is not ground into a fine powder and intimately mixed with the KBr, the IR beam will encounter clumps of the sample. This causes a sloping baseline and poorly resolved peaks because the light scatters unevenly.
Contamination from Water
KBr and NaCl salt plates are highly hygroscopic, meaning they readily absorb water from the atmosphere. Water has very broad, strong IR absorption bands that can easily obscure important peaks in your sample's spectrum. Always use dry materials and store them in a desiccator.
Making the Right Choice for Your Goal
Select your sample preparation method based on the nature of your sample and the amount you have available.
- If your primary focus is conserving a precious or limited sample: Use the ATR technique, as it requires the least amount of material (sub-milligram) and is non-destructive.
- If your primary focus is obtaining a high-quality library spectrum for a solid: Prepare a KBr pellet, carefully aiming for a 0.5-1.0% concentration by mixing 1-2 mg of sample with ~200 mg of KBr.
- If your primary focus is analyzing a pure liquid or a dissolved solid: Create a thin film by placing a single drop between two salt plates.
Ultimately, proper sample preparation is the most critical step to ensure your IR spectrum is both accurate and informative.
Summary Table:
| IR Technique | Typical Sample Amount | Key Consideration |
|---|---|---|
| KBr Pellet (Solids) | 1-2 mg | Mix with ~200 mg KBr for 0.5-1.0% concentration |
| Thin Film (Liquids/Solutions) | A single drop | Create a thin layer between salt plates |
| ATR (Solids/Liquids) | 0.5-1 mg (pinpoint) | Just cover the ATR crystal surface |
Achieve Perfect IR Spectra with the Right Equipment from KINTEK
Struggling with sample preparation or inconsistent results? KINTEK specializes in high-quality lab equipment and consumables designed specifically for reliable infrared spectroscopy. Whether you need durable KBr presses, pristine salt plates, or versatile ATR accessories, we provide the tools for precise, reproducible analysis.
Let us help you optimize your workflow and conserve precious samples. Our experts understand your laboratory's challenges and are ready to recommend the perfect solution for your specific application.
Contact our team today to discuss your IR needs and discover how KINTEK can enhance your laboratory's efficiency and accuracy.
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
People Also Ask
- What are the safety precautions for KBr? Achieve Flawless FTIR Pellet Preparation and Data Accuracy
- Why do we use KBr in IR spectroscopy? Achieve Clear, High-Quality Solid Sample Analysis
- Why KBr is used for IR spectroscopy? The Ideal Medium for Solid Sample Analysis
- Why only KBr is used in IR spectroscopy? The Truth About the Best Material for Your Sample
- How do you prepare a KBr pellet for IR spectroscopy? Master the Key Steps for a Clear Spectrum



















