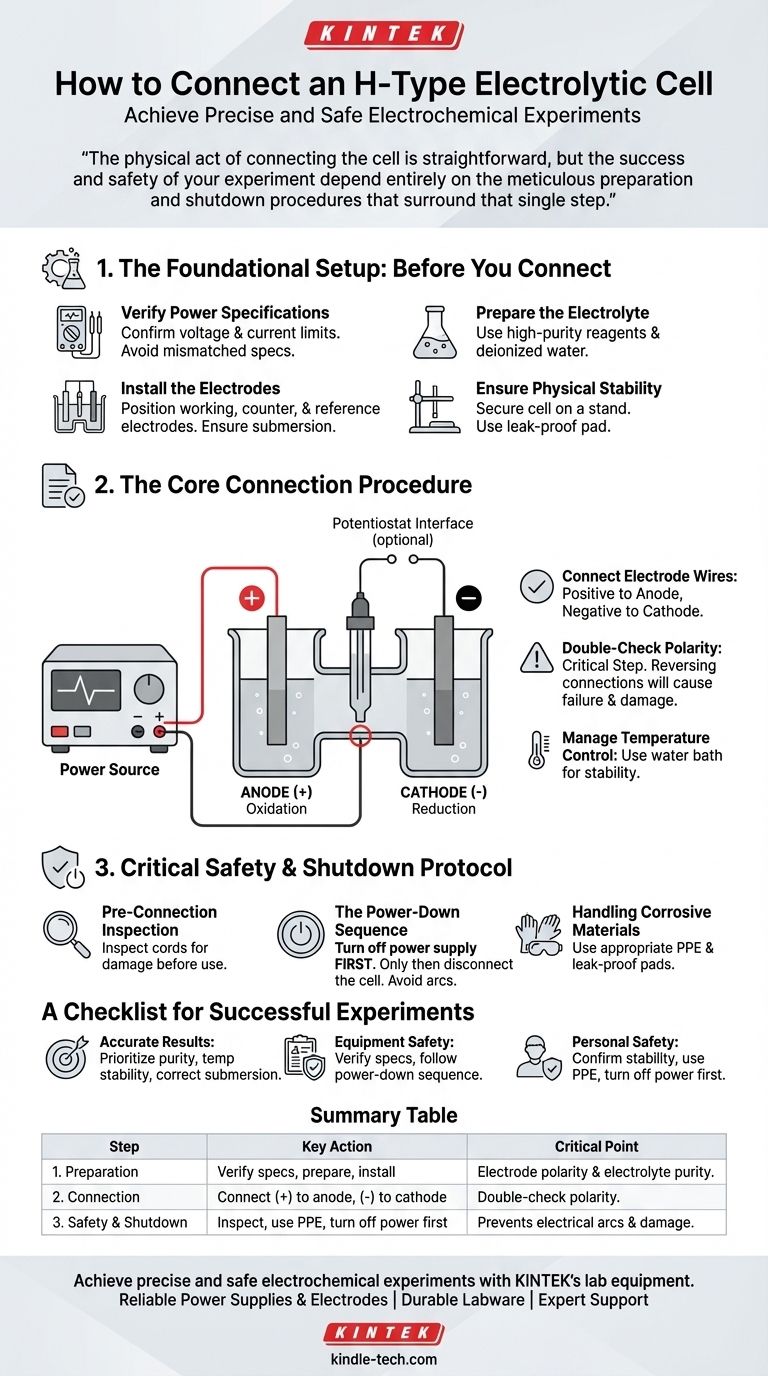
To connect an H-type electrolytic cell, you must attach the positive terminal of the power source to the anode (where oxidation occurs) and the negative terminal to the cathode (where reduction occurs). This polarity is critical, as reversing the connections will cause the experiment to fail and may damage your electrodes or the cell itself. Always verify the power supply's voltage and current settings are appropriate for your specific experiment before making any connections.
The physical act of connecting the cell is straightforward, but the success and safety of your experiment depend entirely on the meticulous preparation and shutdown procedures that surround that single step.

The Foundational Setup: Before You Connect
Proper preparation is the most critical phase for ensuring reliable data and safe operation. Rushing this stage is a common source of experimental error.
Verify Power Specifications
Before anything else, confirm that your power source's voltage and current capabilities are within the safe operating limits for your specific electrodes and cell. Mismatched specifications are a primary cause of equipment damage.
Prepare the Electrolyte
Use high-purity chemical reagents and deionized or distilled water to prepare your electrolyte. Impurities can significantly alter experimental results. Pour the prepared solution into the cell, ensuring you do not exceed the maximum volume.
Install the Electrodes
Position the three electrodes (working, counter, and reference) correctly within the reaction vessel. Ensure they are fully submerged in the electrolyte but that the connecting rods at the top remain dry. Maintain appropriate spacing between them as required by your experimental design.
Ensure Physical Stability
Place the electrolytic cell securely on the base of a stand and tighten any fixing knobs. The cell must be stable and vertical to prevent spills or movement. For corrosive electrolytes, always place a leak-proof pad underneath the cell as a precaution.
The Core Connection Procedure
With the cell prepared, the connection to the power source or electrochemical workstation is a precise, deliberate process.
Connect Electrode Wires
Attach the electrode wires to the corresponding ports on your power source or workstation. The positive lead connects to the anode, and the negative lead connects to the cathode.
Double-Check Polarity
This is the most crucial step. Re-verify that the positive terminal is connected to the anode and the negative terminal is connected to the cathode. Reversing these connections will reverse the electrochemical reactions.
Manage Temperature Control
If your experiment requires a stable temperature, place the cell into a constant temperature water bath. Connect the inlet and outlet pipes to ensure proper circulation before powering on the system.
Critical Safety and Shutdown Protocol
Mistakes during setup or shutdown can compromise safety and equipment integrity. Following a strict protocol is non-negotiable.
Pre-Connection Inspection
Always inspect all power cords and connection lines for any signs of damage or wear before use. A frayed or damaged cord is a significant safety hazard.
The Power-Down Sequence
At the conclusion of your experiment, the shutdown order is critical. First, turn off the power supply. Only after the power is completely off should you disconnect the electrolytic cell. Disconnecting a live cell can generate an electrical arc.
Handling Corrosive Materials
When working with corrosive electrolytes, always use appropriate personal protective equipment (PPE). The use of a leak-proof pad under the cell protects your work surface from accidental spills.
A Checklist for Successful Experiments
Your approach to the setup should be dictated by your primary experimental objective.
- If your primary focus is ensuring accurate results: Prioritize the purity of your electrolyte, the stability of the cell's temperature, and the correct submersion of the electrodes.
- If your primary focus is ensuring equipment safety: Your first step should always be to verify power supply specifications and meticulously follow the correct power-down sequence.
- If your primary focus is ensuring personal safety: Confirm the cell's physical stability, use a leak-proof pad for corrosive materials, and never disconnect the cell before turning off the power.
A methodical and disciplined setup is the foundation of reliable electrochemical research.
Summary Table:
| Step | Key Action | Critical Point |
|---|---|---|
| 1. Preparation | Verify power specs, prepare electrolyte, install electrodes. | Ensure electrode polarity and electrolyte purity. |
| 2. Connection | Connect positive to anode, negative to cathode. | Double-check polarity to avoid failed experiments. |
| 3. Safety & Shutdown | Inspect equipment, use PPE, turn off power before disconnecting. | Prevents electrical arcs and damage. |
Achieve precise and safe electrochemical experiments with KINTEK's lab equipment.
Whether you are setting up H-type cells for research or routine analysis, using the right tools is essential for success. KINTEK specializes in providing high-quality laboratory equipment and consumables designed for reliability and accuracy.
Let us support your work:
- Reliable Power Supplies & Electrodes: Ensure stable current and voltage for consistent results.
- Durable Labware: From electrolytic cells to safety equipment, built to withstand demanding conditions.
- Expert Support: Get guidance on selecting the right equipment for your specific experimental needs.
Enhance your lab's capabilities and safety — Contact our experts today to find the perfect solution for your electrochemical applications!
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What is the function of an H-type exchangeable membrane electrolytic cell? Master Precise Reaction Control
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab



















