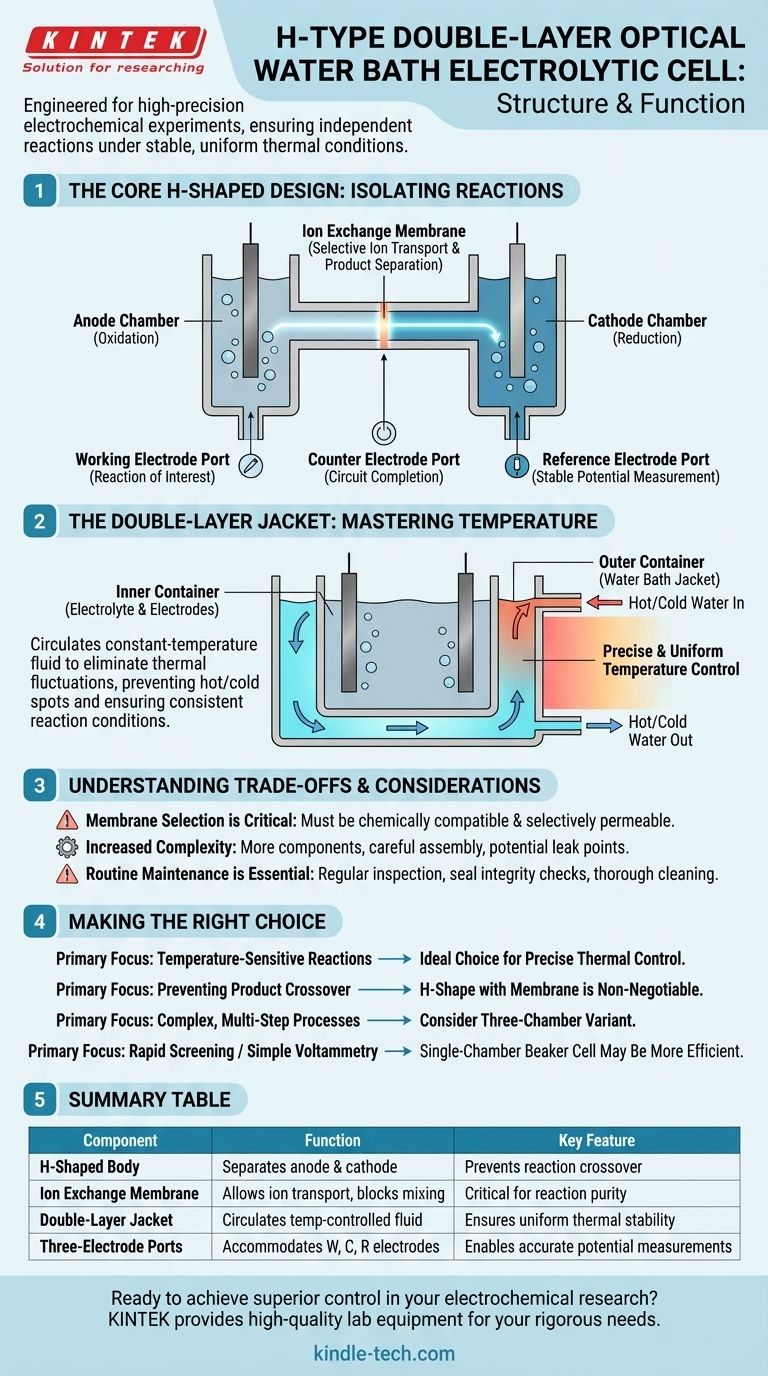
The H-type double-layer optical water bath electrolytic cell is a specialized piece of laboratory equipment designed for high-precision electrochemical experiments. Its structure is defined by three key features: an H-shaped body that separates the anode and cathode, a double-layer jacket for external temperature control, and accommodations for a standard three-electrode system. This design ensures that reactions at each electrode occur independently under stable, uniform thermal conditions.
The fundamental purpose of this cell's complex structure is to achieve experimental control and reproducibility. The H-shape isolates electrochemical processes, while the double-layer jacket eliminates temperature fluctuations, two of the most significant variables in sensitive electrochemical studies.

The Core H-Shaped Design: Isolating Reactions
The "H" in the name directly describes the cell's physical shape, which is critical for separating the electrochemical reactions.
Anode and Cathode Chambers
The cell is physically divided into two distinct, vertical chambers. One chamber houses the anode and its associated reactions (oxidation), while the other houses the cathode and its reactions (reduction).
The Ion Exchange Membrane
These two chambers are connected at the horizontal crossbar of the "H" by a fixture that holds a replaceable ion exchange membrane. This membrane is the key to the design; it prevents the bulk mixing of electrolytes and reaction products from each chamber while still allowing the necessary transport of ions to maintain charge neutrality and complete the electrical circuit.
The Three-Electrode System
This cell is designed to be used with a three-electrode system. This standard electrochemical setup includes a working electrode (where the reaction of interest occurs), a counter electrode (which completes the circuit), and a reference electrode (which provides a stable potential for accurate measurements). The cell body includes apertures to properly position these electrodes.
The Double-Layer Jacket: Mastering Temperature
The second defining feature is the double-layer construction, which acts as a water jacket for precise thermal management.
Inner and Outer Containers
The cell consists of an inner container, where the electrolyte and electrodes are held, and a sealed outer container. The space between these two layers is designed for the circulation of a constant-temperature liquid from an external water bath.
Precise Temperature Control
By circulating fluid (like hot or cold water) through the outer jacket, the temperature inside the inner cell can be maintained within a very narrow range. This is crucial for mitigating heat generated by the electrolysis itself or insulating the reaction from ambient temperature changes.
Uniform Temperature Distribution
The water bath provides an even temperature field around the entire inner cell. This prevents local "hot spots" or "cold spots" from forming on the electrode surfaces, ensuring reaction conditions are consistent and improving the efficiency and quality of the results.
Understanding the Trade-offs and Common Pitfalls
While powerful, this cell's design introduces complexities that users must manage for successful experiments.
Membrane Selection is Critical
The choice of ion exchange membrane (e.g., Nafion, Selemion) is not trivial. The membrane must be chemically compatible with the electrolyte and selectively permeable to the correct ions. An incorrect choice can lead to failed experiments or contamination.
Increased Complexity
Compared to a simple, single-chamber beaker cell, the H-type cell has more components, requires careful assembly, and presents more potential points of failure, such as leaks in the seals around the membrane.
Routine Maintenance is Essential
The cell requires diligent upkeep. This includes regularly inspecting the glass for damage, checking the integrity of seals, and thoroughly cleaning the inner surfaces to remove residual electrolytes, which could contaminate future experiments.
Making the Right Choice for Your Experiment
Use the cell's structural features to guide your decision on whether it's the appropriate tool for your research goal.
- If your primary focus is on temperature-sensitive reactions: The double-layer water jacket makes this cell the ideal choice for its precise thermal control.
- If your primary focus is preventing product crossover: The H-shape with its separating ion exchange membrane is a non-negotiable feature.
- If your primary focus is analyzing complex, multi-step processes: Consider a three-chamber variant, which adds an intermediate chamber for more advanced experimental setups.
- If your primary focus is rapid screening or simple voltammetry: A less complex single-chamber beaker cell may be a more efficient and cost-effective option.
Understanding this cell's architecture empowers you to design more controlled, accurate, and repeatable electrochemical experiments.
Summary Table:
| Component | Function | Key Feature |
|---|---|---|
| H-Shaped Body | Separates anode and cathode chambers | Prevents reaction crossover |
| Ion Exchange Membrane | Allows ion transport, blocks product mixing | Critical for reaction purity |
| Double-Layer Jacket | Circulates temperature-controlled fluid | Ensures uniform thermal stability |
| Three-Electrode Ports | Accommodates working, counter, and reference electrodes | Enables accurate potential measurements |
Ready to achieve superior control in your electrochemical research?
The H-type double-layer optical water bath electrolytic cell is engineered for precision, reproducibility, and thermal stability—exactly what your sensitive experiments demand. At KINTEK, we specialize in providing high-quality lab equipment, including advanced electrochemical cells, to meet the rigorous needs of research and development laboratories.
Let us help you enhance your experimental outcomes. Our experts can guide you to the right equipment for your specific application, ensuring you get the performance and reliability your work requires.
Contact KINTEK today for a consultation and discover how our solutions can power your next breakthrough.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the standard aperture sizes on the lid of the multifunctional electrolytic cell? Key Ports for Your Electrochemical Setup
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- How can the electrochemical reaction be controlled when using this electrolytic cell? Master Voltage, Current & Electrolyte
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments



















