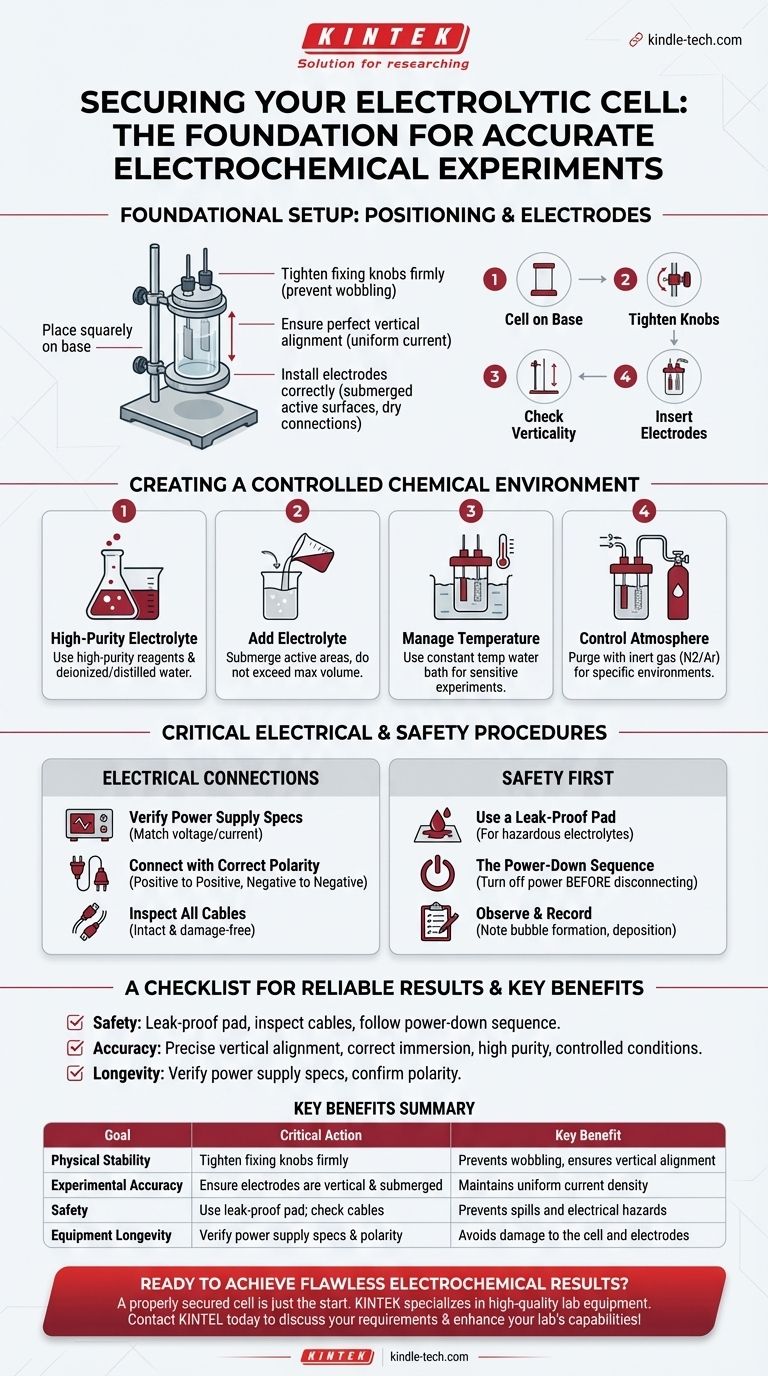
To properly secure an electrolytic cell, you must place it squarely onto the base of the stand and tighten the fixing knobs until it is held firmly. The primary goals are to ensure the cell remains perfectly vertical and does not wobble during the experiment, which is critical for consistent results.
Securing the cell to its stand is the first step in a larger process. The ultimate goal is not just physical stability, but creating a controlled, safe, and repeatable environment to ensure the integrity of your electrochemical experiment from start to finish.

Foundational Setup: Positioning the Cell and Electrodes
A stable physical setup is the bedrock of any reliable experiment. Errors introduced at this stage can compromise all subsequent data.
Place the Cell on the Base
Position the electrolytic cell firmly on the base of the laboratory stand. This provides a stable, level foundation for the entire apparatus.
Tighten the Fixing Knobs
Use the provided fixing knobs or clamps to secure the cell. Tighten them enough to prevent any movement or wobbling, but avoid over-torquing, which could damage the cell's housing.
Ensure Vertical Alignment
A perfectly vertical cell ensures that the distance between electrodes remains constant from top to bottom. This is crucial for maintaining a uniform current density across the electrode surfaces, preventing skewed results.
Install Electrodes Correctly
Insert the working, counter, and reference electrodes into their designated ports. Ensure they are spaced appropriately and are not touching. The active surfaces of the electrodes must be fully submerged in the electrolyte, but the conductive rods or pins at the top must remain dry.
Creating a Controlled Chemical Environment
The cell's contents and surrounding conditions are just as important as its physical stability. Control over the chemical environment is non-negotiable for accuracy.
Prepare High-Purity Electrolyte
Use high-purity reagents and deionized or distilled water to prepare your electrolyte. Impurities can introduce unwanted side reactions and invalidate your results.
Add the Electrolyte
Carefully pour the prepared electrolyte into the cell. Make sure the liquid level is high enough to fully submerge the active areas of all electrodes but does not exceed the cell's maximum volume line.
Manage Temperature
If your experiment is temperature-sensitive, place the cell in a constant temperature water bath. Connect the inlet and outlet pipes to ensure stable, circulating water maintains the target temperature throughout the experiment.
Control the Atmosphere
For experiments requiring a specific atmosphere (like an inert environment), purge the sealed cell with the required gas, such as nitrogen or argon, to remove oxygen and other reactive gases before beginning your measurement.
Understanding the Electrical Connections
Incorrect electrical connections are a common source of experimental failure and can pose significant safety risks. A methodical approach is essential.
Verify Power Supply Specifications
Before connecting anything, confirm that your power source's voltage and current capabilities are within the acceptable range for your electrolytic cell and electrodes. Mismatched specifications can cause irreversible damage.
Connect with Correct Polarity
Connect the electrode leads to the corresponding terminals on the electrochemical workstation or power supply. Always double-check that the positive and negative terminals are connected correctly. Reversing the polarity can damage the cell and will cause the experiment to fail.
Inspect All Cables
Perform a quick visual inspection of all power cords and connection lines. Ensure they are intact and free of damage to prevent electrical shorts or other safety hazards.
Critical Safety and Operational Procedures
Safety is not just a checklist; it is an integrated part of the experimental procedure. Certain steps are mandatory to protect both the user and the equipment.
Use a Leak-Proof Pad
If you are working with a corrosive or hazardous electrolyte, always place a leak-proof pad or secondary containment tray under the electrolytic cell. This simple step can prevent a small leak from becoming a major safety incident or damaging the lab bench.
The Power-Down Sequence
After the experiment is complete, always turn off the power supply first. Only after the power is off should you disconnect the cables from the electrolytic cell. Disconnecting a live circuit can generate an electrical arc, posing a serious safety risk.
Observe and Record
During the experiment, carefully observe the phenomena at the electrode surfaces, such as bubble formation or material deposition. Correlating these physical observations with your recorded data is key to a full analysis.
A Checklist for Reliable Results
Your specific priorities will determine where to focus the most attention during setup. Use this guide to align your procedure with your goal.
- If your primary focus is safety: Prioritize using a leak-proof pad for hazardous liquids, inspecting all cables, and strictly following the power-down sequence to prevent electrical arcs.
- If your primary focus is experimental accuracy: Ensure precise vertical alignment, correct electrode immersion depth, use of high-purity electrolyte, and strict control over temperature and atmosphere.
- If your primary focus is equipment longevity: Double-check that the power supply's voltage is compatible with the cell's rating and always confirm the correct polarity before turning on the power.
A methodical and deliberate setup is the foundation of successful and safe electrochemical research.
Summary Table:
| Setup Goal | Critical Action | Key Benefit |
|---|---|---|
| Physical Stability | Tighten fixing knobs firmly | Prevents wobbling, ensures vertical alignment |
| Experimental Accuracy | Ensure electrodes are vertical and submerged | Maintains uniform current density |
| Safety | Use leak-proof pad; check cables | Prevents spills and electrical hazards |
| Equipment Longevity | Verify power supply specs & polarity | Avoids damage to the cell and electrodes |
Ready to achieve flawless electrochemical results? A properly secured cell is just the start. KINTEK specializes in high-quality lab equipment and consumables for all your laboratory needs. Our experts can help you select the right electrolytic cells and stands to ensure your experiments are safe, accurate, and repeatable.
Contact KINTEL today to discuss your specific requirements and enhance your lab's capabilities!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What are the standard aperture specifications of the electrolytic cell? Key Sizes for Your Electrochemical Setup
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the standard aperture sizes on the lid of the multifunctional electrolytic cell? Key Ports for Your Electrochemical Setup
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy



















