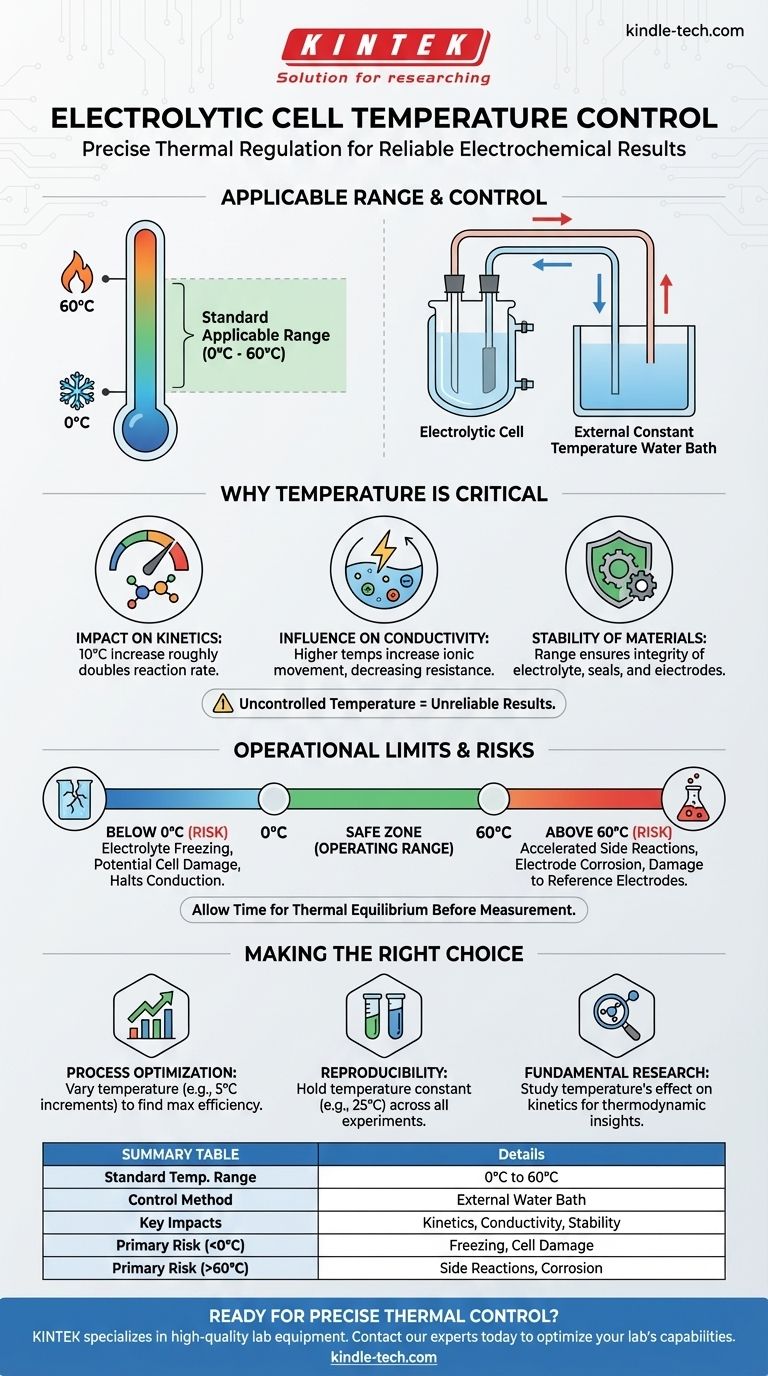
In practice, the standard applicable temperature range for an electrolytic cell is from 0°C to 60°C. Temperature is managed not by an internal heater, but by connecting the cell to an external constant temperature water bath. This setup allows for precise thermal regulation to meet the specific demands of different electrochemical experiments.
The core challenge isn't just knowing the temperature range, but understanding that precise temperature control is a fundamental variable, just as critical as voltage or current, for achieving accurate and repeatable electrochemical results.

Why Temperature Is a Critical Parameter
Temperature is an active and influential factor in any electrochemical system. Failing to control it means you are leaving a key variable to chance, which can invalidate your results.
Impact on Reaction Kinetics
Every 10°C increase in temperature can roughly double the rate of a chemical reaction. In electrochemistry, this directly affects the speed of electron transfer at the electrode surfaces, influencing current density and the overall efficiency of your process.
Influence on Electrolyte Conductivity
The mobility of ions within the electrolyte is highly dependent on temperature. Higher temperatures decrease the viscosity of the solvent and increase ionic movement, leading to higher conductivity. Uncontrolled temperature fluctuations will cause conductivity to drift, altering the cell's resistance and the measured potential.
Stability of Materials
The specified range of 0°C to 60°C is not arbitrary. It is set to ensure the chemical and physical integrity of the cell's components, including the electrolyte, seals, and electrodes.
The Control Mechanism: The Water Bath
Using an external system is the standard for high-precision applications. This method isolates the heating and cooling from the sensitive electrical environment of the cell.
How It Works
Most research-grade electrolytic cells are "jacketed," meaning they have an outer shell with an inlet and an outlet port. Hoses connect these ports to an external constant temperature water bath, which continuously circulates fluid at a set temperature through the jacket, creating a thermal sleeve around the cell.
Benefits of External Control
This approach provides highly uniform and stable temperature throughout the electrolyte. It also prevents the introduction of electrical noise that an internal heating element might create, which is critical for sensitive measurements like cyclic voltammetry or electrochemical impedance spectroscopy.
Understanding the Operational Limits
Operating outside the recommended 0°C to 60°C range introduces significant risks to both your experiment and your equipment.
The Risk of Operating Below 0°C
The primary danger is the freezing of the aqueous electrolyte. This can cause the volume to expand, potentially cracking the glass cell. Even if the cell doesn't break, a frozen electrolyte halts all ionic conduction, stopping the reaction entirely.
The Risk of Exceeding 60°C
High temperatures can accelerate unwanted side reactions, cause the electrolyte to decompose or evaporate, and speed up the corrosion of your electrodes. Furthermore, many standard reference electrodes can be permanently damaged by excessive heat.
The Need for Thermal Equilibrium
When you set a new temperature, you must allow enough time for the entire cell and its contents to reach thermal equilibrium. Starting a measurement before the system is stable will produce skewed, drifting data as the temperature slowly equalizes.
Making the Right Choice for Your Goal
Controlling temperature is not just about safety; it's about tailoring the experimental conditions to your objective.
- If your primary focus is process optimization: Systematically vary the temperature within the safe range (e.g., in 5°C increments) to find the point of maximum efficiency or product yield.
- If your primary focus is reproducibility: Choose a specific temperature (e.g., 25°C) and use the water bath to hold it constant across all related experiments to ensure your results are comparable.
- If your primary focus is fundamental research: Intentionally study the effect of temperature on the reaction kinetics to understand the underlying thermodynamic and activation parameters of your system.
Mastering your experimental parameters is the foundation of reliable and insightful electrochemical work.
Summary Table:
| Aspect | Details |
|---|---|
| Standard Temperature Range | 0°C to 60°C |
| Control Method | External constant temperature water bath |
| Key Impact of Temperature | Reaction kinetics, electrolyte conductivity, material stability |
| Primary Risk (Below 0°C) | Electrolyte freezing, potential cell damage |
| Primary Risk (Above 60°C) | Accelerated side reactions, electrode corrosion |
Ready to achieve precise thermal control in your electrochemical experiments?
KINTEK specializes in high-quality lab equipment, including electrolytic cells and compatible temperature control systems. Ensuring precise and stable temperature is crucial for the accuracy and reproducibility of your research. Our solutions are designed to help you master this critical variable.
Let us help you optimize your lab's capabilities. Contact our experts today to discuss your specific needs!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments



















