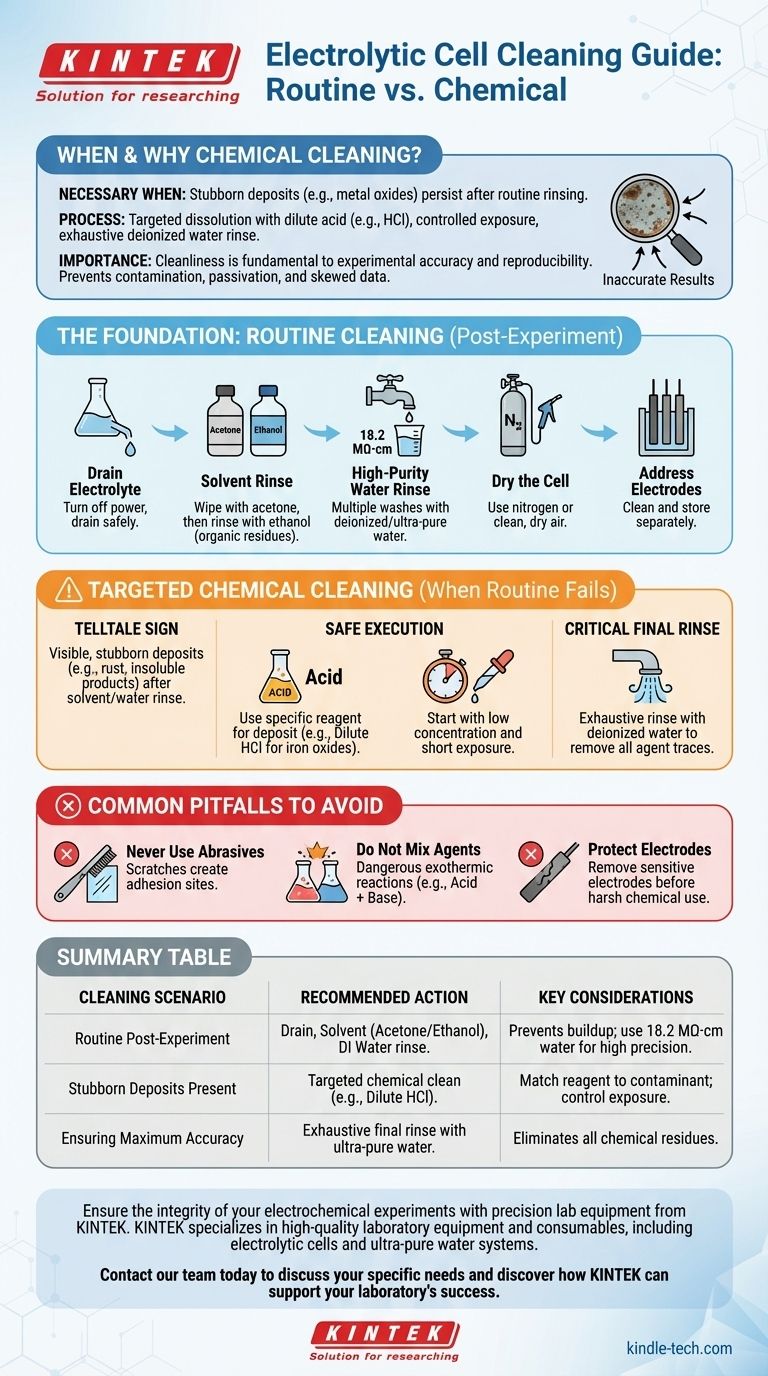
In short, chemical cleaning is necessary when stubborn deposits, such as metal oxides, cannot be removed by simple rinsing with solvents or water. The process involves carefully selecting a chemical reagent like dilute acid to dissolve the specific contaminant, controlling the exposure time to prevent damage, and finishing with an exhaustive rinse using deionized water to remove all chemical residues.
The cleanliness of your electrolytic cell is not a trivial housekeeping task; it is a fundamental variable in your experiment. A disciplined, tiered cleaning strategy—from routine rinsing to targeted chemical intervention—is the most effective way to prevent contamination and ensure the accuracy and reproducibility of your results.

The Foundation: Routine Post-Experiment Cleaning
Before considering aggressive chemical treatments, a consistent and thorough routine cleaning process should be your first line of defense. This prevents the buildup that necessitates harsher methods.
Step 1: Drain the Electrolyte
Immediately after an experiment concludes, turn off the power source. Carefully drain the electrolyte from the cell and handle its disposal according to your institution's safety and environmental regulations.
Step 2: Perform an Initial Solvent Rinse
To remove the bulk of residual electrolyte and any soluble byproducts, begin with a multi-stage rinse. Start by wiping the inner walls with acetone to address organic residues, followed by a rinse with ethanol.
Step 3: Rinse with High-Purity Water
After the solvent rinse, thoroughly wash the cell multiple times with deionized or ultra-pure water. For high-precision work, water with a resistivity of 18.2 MΩ·cm is the standard, ensuring no ionic contaminants are introduced.
Step 4: Dry the Cell
Gently dry the cell with a stream of inert gas like nitrogen or clean, dry air. This prevents water spots and prepares the cell for storage or immediate reuse.
Step 5: Address the Electrodes
Electrodes should be removed from the cell and cleaned separately according to their specific material requirements. For electrodes prone to oxidation, store them appropriately, such as by immersing them in a designated protective solution.
When to Escalate to Chemical Cleaning
Chemical cleaning is a targeted intervention, not a routine procedure. It should only be employed when standard cleaning fails.
The Telltale Sign: Persistent Deposits
The primary indicator for chemical cleaning is the presence of visible, stubborn deposits that remain after thorough solvent and water rinsing. These are often metal oxides (like rust from iron contaminants) or other insoluble reaction products firmly adhered to the cell walls or electrodes.
The Impact of Contamination
These deposits are not merely cosmetic. They can passivate electrode surfaces, increase cell resistance, or leach ions into your electrolyte, leading to inaccurate measurements, skewed data, and poor experimental reproducibility.
Executing a Safe and Effective Chemical Clean
When chemical cleaning is deemed necessary, it must be performed with precision and care to resolve the contamination without damaging the apparatus.
Match the Reagent to the Deposit
The core principle is to use a chemical that specifically dissolves the contaminant. For example, a dilute hydrochloric acid (HCl) solution is effective for removing common iron oxides. Always research the appropriate reagent for the specific deposit you are targeting.
Control Concentration and Duration
To avoid damaging the cell's glass or sensitive electrode materials, always start with a low concentration of the cleaning agent and a short exposure time. You can gradually increase these parameters if necessary, but minimal effective exposure is the goal.
The Critical Final Rinse
After the chemical treatment, it is absolutely essential to remove all traces of the cleaning agent. Rinse the cell exhaustively with a large volume of deionized water until you are confident no residue remains, as any lingering chemical will contaminate subsequent experiments.
Common Pitfalls to Avoid
Improper cleaning techniques can be more destructive than the contamination itself. Adhering to these warnings is critical for safety and equipment longevity.
Never Use Abrasives
Do not use metal brushes or other abrasive tools to scrub the inside of a glass cell. These will inevitably scratch the surface, creating sites where contaminants can more easily adhere in the future and potentially compromising the cell's structural integrity.
Do Not Mix Cleaning Agents
Never mix different cleaning chemicals, especially acids and bases (e.g., nitric acid and sodium hydroxide). This can trigger a dangerous and uncontrolled exothermic reaction, posing a significant safety hazard.
Protect Your Electrodes
Unless the electrode itself is the target of the cleaning, it should be removed from the cell before introducing aggressive chemical agents that could corrode or permanently damage its surface.
Making the Right Choice for Your Goal
Your cleaning strategy should be tailored to the specific needs of your work, balancing efficiency with the demand for analytical precision.
- If your primary focus is routine, post-experiment use: A consistent protocol of draining, solvent rinsing (acetone, then ethanol), and a final deionized water wash is sufficient to prevent contaminant buildup.
- If your primary focus is removing visible, stubborn deposits: Escalate to a targeted chemical clean, carefully matching the reagent to the contaminant and minimizing exposure time and concentration.
- If your primary focus is ensuring maximum data accuracy: Always conclude any cleaning procedure with a final, thorough rinse using ultra-pure (18.2 MΩ·cm) water to eliminate all potential sources of contamination.
A disciplined approach to cell maintenance is the foundation of reliable and reproducible electrochemical science.
Summary Table:
| Cleaning Scenario | Recommended Action | Key Considerations |
|---|---|---|
| Routine Post-Experiment | Drain electrolyte, rinse with acetone/ethanol, then deionized water. | Prevents buildup; use 18.2 MΩ·cm water for high precision. |
| Stubborn Deposits Present | Targeted chemical clean with a reagent like dilute HCl. | Match reagent to contaminant; control exposure time and concentration. |
| Ensuring Maximum Accuracy | Exhaustive final rinse with ultra-pure water after any cleaning. | Removes all chemical residues to prevent contamination of future experiments. |
Ensure the integrity of your electrochemical experiments with precision lab equipment from KINTEK.
Proper cell maintenance is critical for accurate, reproducible results. KINTEK specializes in supplying high-quality laboratory equipment and consumables, including electrolytic cells and ultra-pure water systems, designed to meet the rigorous demands of your research.
Let our experts help you build a reliable lab environment. Contact our team today to discuss your specific needs and discover how KINTEK can support your laboratory's success.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy
- What is the overall structure of the H-type double-layer optical water bath electrolytic cell? Precision Design for Controlled Experiments
- How can the electrochemical reaction be controlled when using this electrolytic cell? Master Voltage, Current & Electrolyte
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments



















