Conducting an experiment with an electrolytic cell requires strict adherence to safety protocols. The primary dangers involve electric shock from electrodes, chemical burns from the electrolyte, and fire or explosion from flammable gases produced during the reaction. Therefore, you must avoid direct contact with energized components, keep all flammable materials away from the apparatus, and ensure the entire process is actively monitored.
An electrolytic cell introduces a unique combination of electrical and chemical hazards. The core principle of safety is to manage these intersecting risks through meticulous preparation, constant vigilance during operation, and proper post-experiment cleanup.

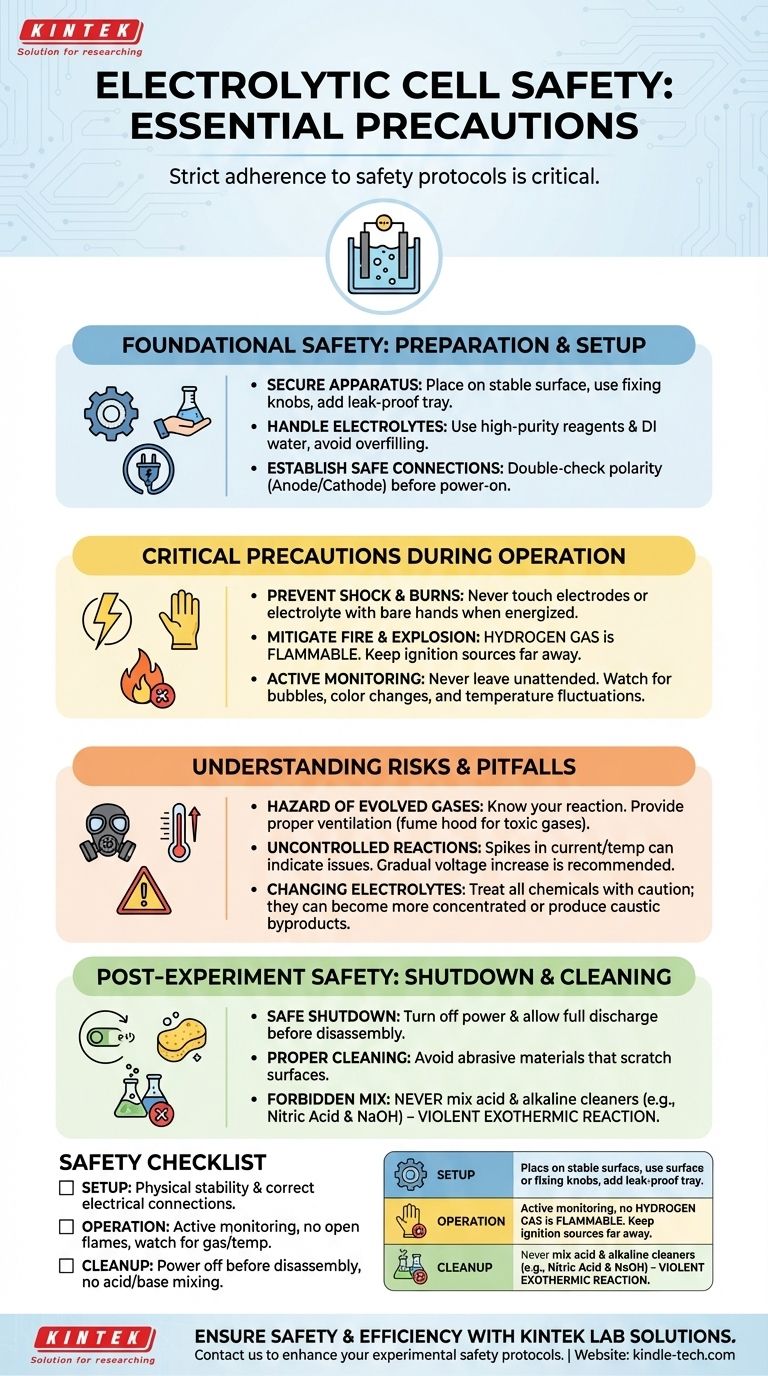
Foundational Safety: Preparation and Setup
Proper setup is the first line of defense against accidents. A stable and correctly configured apparatus minimizes the risk of spills, shorts, and unexpected reactions.
Securing the Physical Apparatus
Place the electrolytic cell on a stable, level surface, ideally on the base of a stand. Use the fixing knobs to ensure the cell is positioned securely and does not wobble.
If you are using a corrosive electrolyte, place a leak-proof pad or a secondary containment tray underneath the cell to catch any potential spills.
Handling Electrolytes Correctly
Always use high-purity chemical reagents and deionized or distilled water to prepare your electrolyte. Impurities can lead to unwanted side reactions and unpredictable results.
When filling the cell, carefully pour the electrolyte and ensure the volume does not exceed the marked maximum capacity to prevent overflow during the experiment.
Establishing Safe Electrical Connections
Before turning on any power, ensure the electrolytic cell is correctly connected to the power supply and any measurement instruments. Double-check that the positive (anode) and negative (cathode) terminals are connected according to your experimental diagram.
Critical Precautions During Operation
Once the experiment is running, your focus must shift to active monitoring and maintaining a safe environment around the cell.
Preventing Electrical Shock and Chemical Burns
Never touch the electrodes or the electrolyte with your bare hands once the power supply is on. This is the most direct way to receive an electric shock or a chemical burn.
Mitigating Fire and Explosion Hazards
Electrolysis often produces gases. The electrolysis of water, for example, produces hydrogen and oxygen. Hydrogen gas is highly flammable.
For this reason, you must strictly prohibit open flames, sparks, or other ignition sources near the electrolytic cell to prevent a fire or explosion.
The Importance of Active Monitoring
An experiment should never be left unattended. Closely monitor the working state of the cell throughout the process.
Look for key indicators such as bubble formation on the electrode surfaces, changes in the color of the electrolyte, and any fluctuations in temperature. Promptly identifying an abnormality is critical to preventing a larger issue.
Understanding the Risks and Pitfalls
A safe experiment requires understanding not just the rules, but the reasons behind them. Awareness of the underlying hazards is crucial.
The Hazard of Evolved Gases
Be aware of the specific gases your reaction produces. While hydrogen is a fire risk, other reactions can produce toxic gases like chlorine. Proper ventilation is essential, and in some cases, experiments should only be conducted inside a fume hood.
The Risk of Uncontrolled Reactions
Without active monitoring, reaction conditions can spiral out of control. A gradual increase in voltage is recommended at the start. Spikes in current or temperature can indicate a short circuit or an unexpectedly vigorous reaction, which could damage the equipment or cause a hazardous spill.
Assuming All Electrolytes Are Benign
The electrolyte itself can change during the experiment, becoming more concentrated or producing caustic byproducts. Treat all chemicals involved with appropriate caution from start to finish.
Post-Experiment Safety: Shutdown and Cleaning
Safety procedures do not end when you stop collecting data. Proper shutdown and cleaning are essential for personal safety and for maintaining the integrity of the equipment.
Safe Shutdown Procedure
Always turn off the power supply and allow the system to fully discharge before you begin to dismantle the apparatus. Never disconnect wires while the power is on.
Proper Cleaning Protocols
When cleaning the cell, never use metal brushes or other abrasive materials that can scratch the surfaces. Scratches can weaken the structure and become sites for contamination in future experiments.
Crucially, it is forbidden to mix acid and alkaline cleaning agents, such as nitric acid and sodium hydroxide. This combination can cause a dangerous and violent exothermic (heat-producing) reaction.
A Safety Checklist for Your Experiment
Use these points to guide your actions at each stage of the experiment.
- If you are setting up the experiment: Prioritize physical stability and correct electrical connections before introducing any power.
- If you are running the experiment: Focus on active monitoring for gas evolution and temperature changes, and keep all ignition sources far away.
- If you are cleaning up: Ensure the power is off before disassembly and never mix acid and base cleaners.
A methodical and safety-conscious approach is the hallmark of a successful and responsible scientific investigation.
Summary Table:
| Stage | Key Safety Focus | Critical Actions |
|---|---|---|
| Setup | Physical Stability & Correct Connections | Secure cell on stable surface; double-check anode/cathode connections. |
| Operation | Active Monitoring & Hazard Mitigation | No touching electrodes; keep flames away; monitor for gas/temperature changes. |
| Cleanup | Safe Shutdown & Proper Cleaning | Turn off power before disassembly; never mix acid and base cleaners. |
Ensure your lab's electrolysis experiments are conducted safely and efficiently. KINTEK specializes in high-quality lab equipment and consumables, including durable electrolytic cells and safe containment solutions. Our products are designed with safety and precision in mind, helping you mitigate risks and achieve reliable results. Contact our experts today to find the perfect equipment for your laboratory's specific needs and enhance your experimental safety protocols.
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What are the advantages of using a PTFE electrochemical cell in actinide research? Ensure Precise Corrosion Data
- How does an electrochemical synthesis system facilitate MOF thin film preparation? Precision Engineering for Sensors
- What are the key material properties and structural features of an all-PTFE electrolytic cell? Achieve Unmatched Purity in Harsh Electrochemical Environments
- What are the advantages of using a three-electrode system in an electrolytic cell? Ensure Precision Corrosion Testing.
- How is electrodeposition different from electrochemical deposition? The Terms Are Interchangeable
- How do customized three-electrode electrolytic cell systems facilitate long-term stability testing for catalysts?
- What is the electrolysis method of water treatment? A Guide to Electrochemical Purification
- How does a three-electrode electrolytic cell system control MnO2 nanosheet loading? Achieve Micro-Level Precision



















