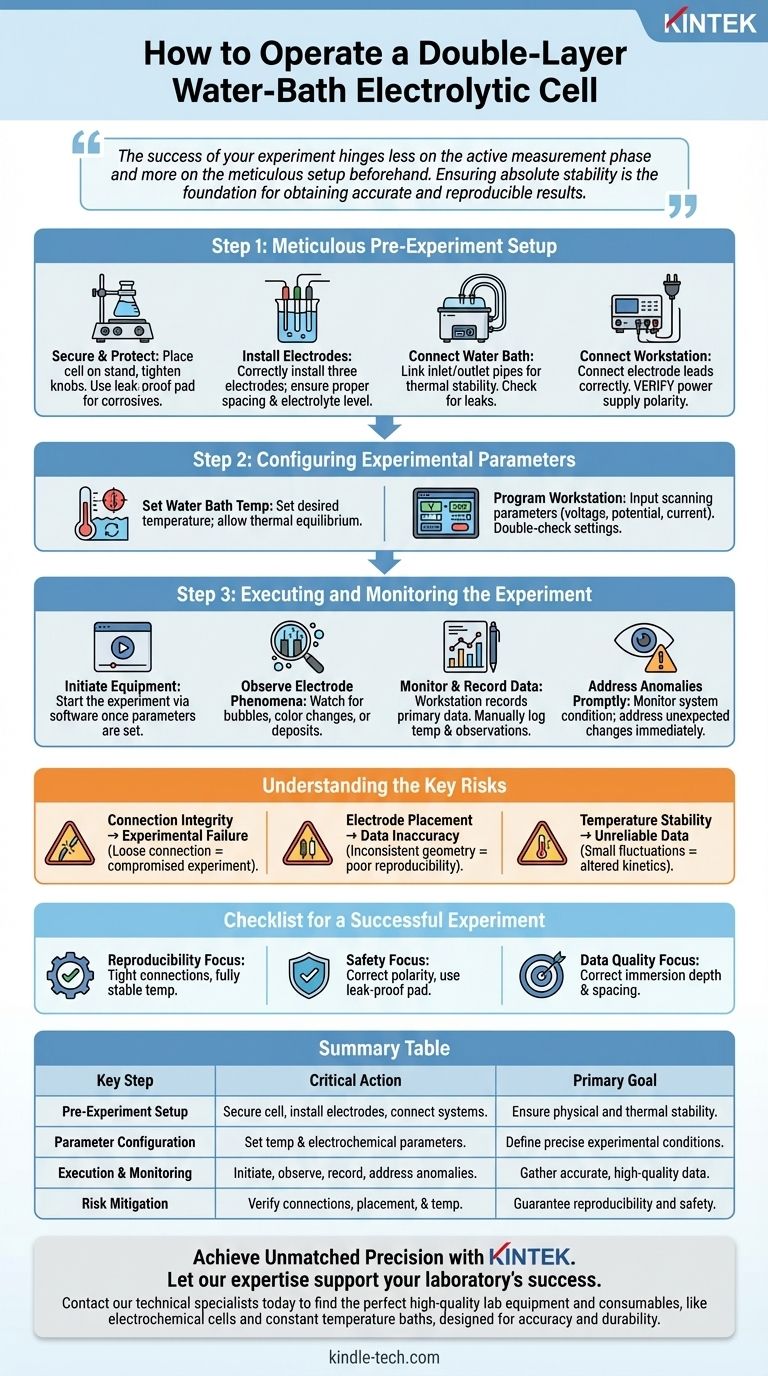
To operate a double-layer water-bath electrolytic cell, you must follow a precise sequence of connecting the equipment, configuring the parameters, and then executing the experiment. This involves correctly linking the cell to the constant temperature water bath and the electrochemical workstation, setting the desired temperature and electrical parameters, and then actively monitoring the electrode surfaces and recording all relevant data.
The success of your experiment hinges less on the active measurement phase and more on the meticulous setup beforehand. Ensuring absolute stability in temperature, electrical connections, and physical placement is the foundation for obtaining accurate and reproducible results.

Step 1: Meticulous Pre-Experiment Setup
Before any power is applied, a methodical physical and electrical setup is essential. Errors at this stage are the most common cause of failed experiments.
Secure the Cell Physically
First, place the electrolytic cell onto the base of a laboratory stand. Tighten the fixing knobs to ensure the cell is positioned vertically and remains completely stable without any wobble.
If you are working with a corrosive electrolyte, it is critical to place a leak-proof pad directly underneath the cell to protect your work surface and equipment.
Install the Electrodes Correctly
Install the three electrodes (working, counter, and reference) into the reaction vessel. Pay close attention to the spacing between them, as this can significantly impact your results.
Next, add the electrolyte to the vessel. The level should be high enough to fully submerge the active surfaces of all electrodes but low enough to ensure the conductive electrode rods themselves are not immersed.
Connect the Temperature Control System
Your primary goal is thermal stability. Connect the inlet and outlet pipes from the constant temperature water bath to the corresponding ports on the electrolytic cell's outer jacket.
Ensure all connections are secure and sealed to prevent water leaks and to guarantee proper circulation, which is vital for maintaining a uniform temperature.
Connect to the Electrochemical Workstation
Connect the electrode leads from the workstation to the corresponding terminals on the electrodes. This connection must be correct to avoid damaging your equipment or invalidating the experiment.
Critically, verify the connection to the main power supply. Reversing the positive and negative terminals can lead to immediate experimental failure or permanent damage to the cell.
Step 2: Configuring Experimental Parameters
With the physical system fully assembled, you can now define the conditions for your experiment.
Set the Water Bath Temperature
Turn on the constant temperature water bath and set it to your desired experimental temperature. Allow sufficient time for the system to circulate and for the electrolyte within the cell to reach thermal equilibrium.
Program the Electrochemical Workstation
Turn on the electrochemical workstation. Carefully input the required parameters for your experiment, such as the scanning voltage range, potential, or desired current. Double-check all settings before proceeding.
Step 3: Executing and Monitoring the Experiment
This is the active data-gathering phase. Vigilant observation is key.
Initiate the Equipment
Once the temperature is stable and the parameters are set, you can begin the experiment via the workstation's software.
Observe Electrode Phenomena
Closely watch the surfaces of the electrodes throughout the run. Look for key indicators of electrochemical reactions, such as the generation of bubbles, changes in color, or the formation of deposits.
Monitor and Record Data
The workstation will automatically record primary data like potential, current, and time. You should also manually log the temperature and any qualitative observations made during the run.
Address Anomalies Promptly
Continuously monitor the overall condition of the electrolyte and the system. If you notice any unexpected changes or problems, address them immediately to salvage the experimental run if possible.
Understanding the Key Risks
A successful outcome depends on anticipating and mitigating potential points of failure.
Connection Integrity vs. Experimental Failure
A loose gas tube, a leaking water-bath connection, or an insecure electrode wire can compromise the entire experiment. Every connection point is a potential source of error and must be verified.
Electrode Placement vs. Data Accuracy
The geometry of your electrodes—their spacing and immersion depth—directly influences the electric field and mass transport within the cell. Inconsistent placement between runs is a major source of poor reproducibility.
Temperature Stability vs. Reaction Rate
The entire purpose of the water bath is to eliminate temperature as an uncontrolled variable. Even small fluctuations in temperature can alter reaction kinetics, leading to inaccurate and unreliable data.
Checklist for a Successful Experiment
Use this checklist to align your procedure with your primary objective.
- If your primary focus is reproducibility: Double-check that all connections are tight and ensure the electrolyte temperature is fully stable before initiating each run.
- If your primary focus is safety: Always verify correct power supply polarity and use a leak-proof pad when working with corrosive electrolytes.
- If your primary focus is data quality: Confirm that electrode immersion depth and spacing are correct and consistent with previous experiments.
A methodical and careful approach at every stage is the best guarantee for reliable and insightful results.
Summary Table:
| Key Step | Critical Action | Primary Goal |
|---|---|---|
| Pre-Experiment Setup | Secure cell, install electrodes, connect water bath & workstation. | Ensure physical and thermal stability. |
| Parameter Configuration | Set water bath temperature and electrochemical parameters. | Define precise experimental conditions. |
| Execution & Monitoring | Initiate run, observe electrodes, record data, address anomalies. | Gather accurate, high-quality data. |
| Risk Mitigation | Verify all connections, electrode placement, and temperature stability. | Guarantee reproducibility and safety. |
Achieve Unmatched Precision in Your Electrochemical Research
Mastering the operation of specialized equipment like the double-layer water-bath electrolytic cell is fundamental to generating reliable data. KINTEK specializes in providing high-quality lab equipment and consumables, including electrochemical cells and constant temperature baths, designed for accuracy and durability.
Let our expertise support your laboratory's success. Contact our technical specialists today to find the perfect solutions for your experimental needs and ensure your research is built on a foundation of precision.
Visual Guide
Related Products
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Electrolytic Electrochemical Cell with Five-Port
People Also Ask
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments
- What safety precautions are necessary for temperature control when using a double-layer water-bath electrolytic cell? Ensure Safe and Accurate Experiments
- What are the procedures for after using a double-layer water-bath electrolytic cell? Ensure Equipment Longevity and Data Accuracy
- When is professional repair required for a double-layer water-bath electrolytic cell? Protect Your Lab's Precision and Safety



















