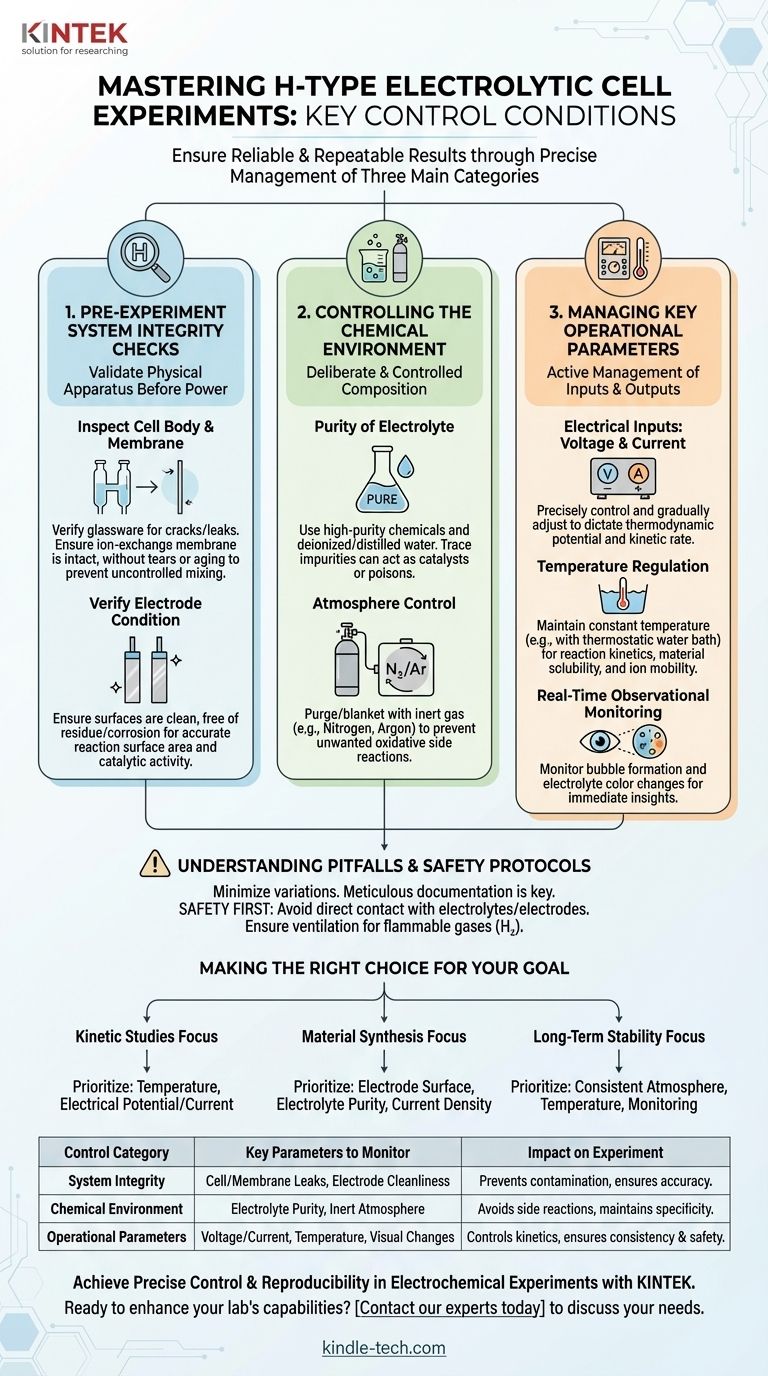
To ensure reliable and repeatable results with an H-type electrolytic cell, you must control three main categories of conditions: the physical integrity of the setup, the chemical purity of the environment, and the precise electrical and thermal parameters during operation. This involves everything from pre-experiment inspection and careful electrolyte preparation to real-time management of voltage, current, and temperature.
The success of an electrochemical experiment is not determined by the reaction alone. It is defined by the rigorous control of the entire system, transforming a simple apparatus into a precise instrument for generating accurate and reproducible data.

1. Pre-Experiment System Integrity Checks
Before any power is applied, you must first validate the integrity of the physical apparatus. A compromised cell guarantees a compromised experiment.
Inspecting the Cell Body and Membrane
The primary function of an H-cell is to separate the anode and cathode chambers. Verify the glassware has no cracks or leaks.
Critically, inspect the ion-exchange membrane. Ensure it is intact, without any tears, holes, or signs of aging and brittleness. A faulty membrane allows uncontrolled mixing of anolyte and catholyte, invalidating your results.
Verifying Electrode Condition
The electrode surface is where the reaction occurs. Its condition is paramount.
Inspect electrodes for cleanliness, ensuring they are free from any previous residue, corrosion, or physical damage. An unclean surface alters the available surface area and catalytic activity, directly impacting reaction rates and outcomes. Polishing or chemical cleaning may be required.
2. Controlling the Chemical Environment
The electrolyte and surrounding atmosphere are active participants in the experiment. Their composition must be deliberate and controlled.
Purity of the Electrolyte
Your results are only as reliable as your reagents. Always prepare the electrolyte using high-purity chemicals and deionized or distilled water.
Trace impurities can act as unwanted catalysts, inhibitors, or result in side reactions that poison your electrodes or contaminate your product.
Atmosphere Control
Many electrochemical reactions are sensitive to oxygen or other components of ambient air.
If your experiment requires an inert environment, you must purge and blanket the relevant chamber with a gas like nitrogen or argon from a cylinder. This prevents unwanted oxidative side reactions from interfering with your primary process.
3. Managing Key Operational Parameters
During the experiment, you must actively manage the inputs and outputs to guide the reaction and ensure consistency.
Electrical Inputs: Voltage and Current
The applied voltage or current is the primary driver of your electrochemical reaction. These parameters must be controlled precisely and adjusted gradually.
They directly dictate the thermodynamic potential and kinetic rate of the reaction. Abrupt changes can lead to unstable conditions and unintended side products.
Temperature Regulation
Reaction kinetics, material solubility, and ion mobility are all highly dependent on temperature.
For any experiment where you want to compare results or understand reaction rates, maintaining a constant temperature is essential. Use a thermostatic water bath to keep the cell at a stable, defined temperature.
Real-Time Observational Monitoring
Your instruments provide data, but your eyes provide context.
Closely monitor the cell during operation. Observe bubble formation on the electrodes, which indicates gas evolution, and watch for any color changes in the electrolyte, which can signify product formation or degradation. These observations help you quickly identify if the experiment is proceeding as expected.
Understanding the Pitfalls and Safety Protocols
Controlling an experiment is also about anticipating and preventing failures, both for data integrity and personal safety.
The Myth of Perfect Control
Recognize that no system is perfect. Minor fluctuations in temperature or voltage can occur. The goal is to minimize these variations and keep them within an acceptable range for your specific application. Documenting your setup meticulously is key to reproducibility.
Safety Is a Critical Control Parameter
An electrolytic cell involves chemical and electrical hazards that must be actively managed.
Never make direct skin contact with the electrolyte or electrodes to prevent chemical burns and electric shock. Furthermore, if your reaction evolves flammable gases like hydrogen (H₂), you must ensure adequate ventilation and keep all open flames or ignition sources far from the apparatus.
Making the Right Choice for Your Goal
The specific parameters you prioritize depend on the objective of your experiment.
- If your primary focus is kinetic studies: Prioritize meticulous control over temperature and the electrical potential/current, as these directly govern reaction rates.
- If your primary focus is material synthesis (electrodeposition): Emphasize electrode surface preparation, electrolyte purity, and consistent current density to ensure a pure and uniform product.
- If your primary focus is long-term stability testing: Focus on maintaining a consistent atmosphere and temperature over extended periods while carefully monitoring for any changes in performance.
By mastering these control points, you ensure that your experimental results are a true reflection of the chemical process you are studying.
Summary Table:
| Control Category | Key Parameters to Monitor | Impact on Experiment |
|---|---|---|
| System Integrity | Cell/membrane leaks, Electrode cleanliness | Prevents contamination, ensures reaction site accuracy |
| Chemical Environment | Electrolyte purity, Inert atmosphere (N₂/Ar) | Avoids side reactions, maintains reaction specificity |
| Operational Parameters | Voltage/current, Temperature, Visual changes (bubbles/color) | Controls reaction kinetics, ensures consistency and safety |
Achieve precise control and reproducibility in your electrochemical experiments with KINTEK.
Our specialized lab equipment and consumables are designed to meet the rigorous demands of H-cell applications, from high-purity electrolytes to reliable temperature control systems. Whether you're focused on kinetic studies, material synthesis, or long-term stability testing, KINTEK provides the tools for success.
Ready to enhance your lab's capabilities? Contact our experts today to discuss your specific needs and discover how our solutions can bring accuracy and efficiency to your research.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What is the function of an H-type exchangeable membrane electrolytic cell? Master Precise Reaction Control
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide



















