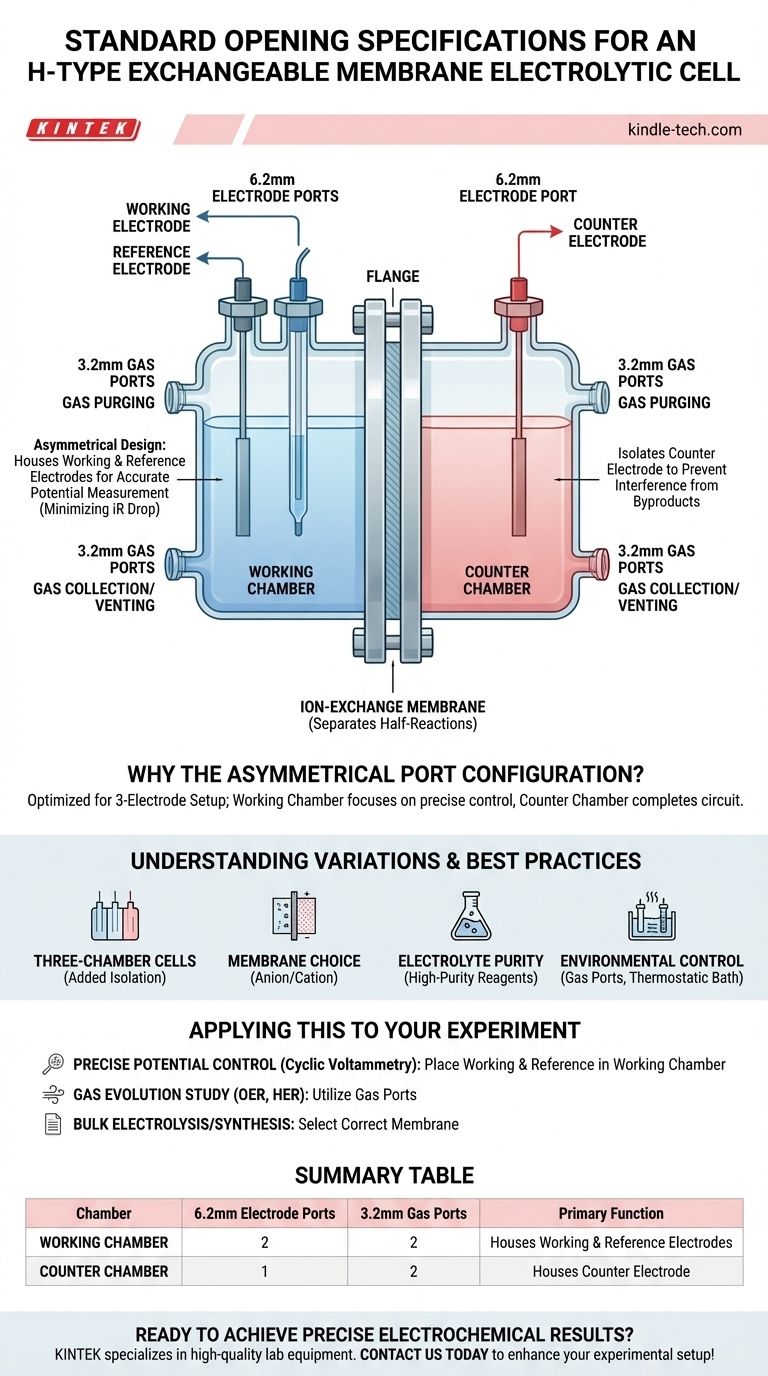
In a standard H-type exchangeable membrane electrolytic cell, the port configuration is designed asymmetrically to accommodate a typical three-electrode setup. One chamber is equipped with two 6.2mm electrode ports and two 3.2mm gas ports. The opposing chamber features a simpler layout with one 6.2mm electrode port and two 3.2mm gas ports.
This asymmetrical design is not arbitrary; it directly supports standard electrochemical practice by allowing the working electrode and reference electrode to be placed in one chamber for accurate potential measurement, while the counter electrode is isolated in the other.
Deconstructing the H-Type Cell's Design
To use an H-type cell effectively, you must understand how its physical form serves its scientific function. The design facilitates the separation and study of electrochemical half-reactions.
The Two Chambers: Anode and Cathode
An H-type cell consists of two distinct glass chambers, typically designated as the anode and cathode compartments.
These chambers are joined by a flange that clamps an ion-exchange membrane. This membrane is the core of the cell's function, allowing specific ions (cations or anions) to pass through while preventing the bulk mixing of the two electrolytes.
The Purpose of Each Port
The openings are standardized to fit common laboratory equipment.
- 6.2mm Electrode Ports: These larger ports are designed to accommodate the electrodes: the working electrode, the counter electrode, and the reference electrode. Their size is standard for common electrode bodies.
- 3.2mm Gas Ports: These smaller ports are used for gas management. This includes purging the electrolyte with an inert gas (like nitrogen or argon) to remove oxygen, or for collecting gaseous products evolved during the reaction (like H₂ or O₂).
Why the Asymmetrical Port Configuration?
The difference in the number of electrode ports between the two chambers is deliberate and highly functional.
The chamber with two 6.2mm electrode ports is the "working chamber." It is designed to house both the working electrode (where the reaction of interest occurs) and the reference electrode. Placing the reference electrode close to the working electrode is critical for minimizing uncompensated resistance (iR drop) and ensuring an accurate measurement of the working electrode's potential.
The chamber with one 6.2mm electrode port is the "counter chamber." It only needs to house the counter electrode, which serves to complete the electrical circuit. Isolating it prevents byproducts from the counter reaction from interfering with the primary reaction at the working electrode.
Understanding Variations and Best Practices
While the 2+1 electrode port design is the most common standard, it's important to be aware of variations and the fundamental principles that guide the cell's use.
Standard vs. Three-Chamber Cells
A less common but important variation is the three-chamber H-type cell. This design introduces a central chamber between the anode and cathode compartments, often to further isolate the reference electrode in its own electrolyte, creating a true salt bridge.
The Critical Role of the Membrane
The choice of membrane is as important as the cell itself. An anion-exchange membrane allows negative ions to pass, while a cation-exchange membrane allows positive ions to pass. Selecting the correct one is fundamental to your experimental goal.
Ensuring Electrolyte Purity
Your results are only as good as your starting materials. Always prepare electrolytes using high-purity chemical reagents and deionized or distilled water. Impurities can introduce unwanted side reactions and invalidate your data.
Maintaining Experimental Control
The cell's design facilitates environmental control. Use the gas ports to create a specific atmosphere if needed. For temperature-sensitive experiments, place the entire H-type cell within a larger thermostatic water bath to maintain a constant, uniform temperature across both chambers.
Applying This to Your Experiment
Your experimental goal determines how you should utilize the cell's features.
- If your primary focus is precise potential control (e.g., cyclic voltammetry): Place your working electrode and reference electrode in the same chamber (the one with two electrode ports) to ensure the most accurate potential measurement.
- If your primary focus is studying gas evolution (e.g., OER, HER): Use the 3.2mm gas ports to purge the electrolyte before the experiment and to safely vent or collect the gaseous products for analysis (e.g., with gas chromatography).
- If your primary focus is bulk electrolysis or product synthesis: Ensure your membrane is chosen correctly to prevent your target product from migrating into the counter chamber and reacting at the counter electrode.
By understanding this standard design, you can configure your electrochemical experiments with precision and confidence.
Summary Table:
| Chamber | 6.2mm Electrode Ports | 3.2mm Gas Ports | Primary Function |
|---|---|---|---|
| Working Chamber | 2 | 2 | Houses Working & Reference Electrodes |
| Counter Chamber | 1 | 2 | Houses Counter Electrode |
Ready to achieve precise electrochemical results?
The standard H-type cell design is critical for separating reactions and ensuring accurate measurements. KINTEK specializes in high-quality lab equipment and consumables, including electrochemical cells and membranes, to serve your laboratory's specific needs.
Let our experts help you select the perfect cell for your application. Contact us today to discuss your requirements and enhance your experimental setup!
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Electrolytic Electrochemical Cell with Five-Port
- FS Electrochemical Hydrogen Fuel Cells for Diverse Applications
People Also Ask
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results
- What is the function of an H-type exchangeable membrane electrolytic cell? Master Precise Reaction Control
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control



















