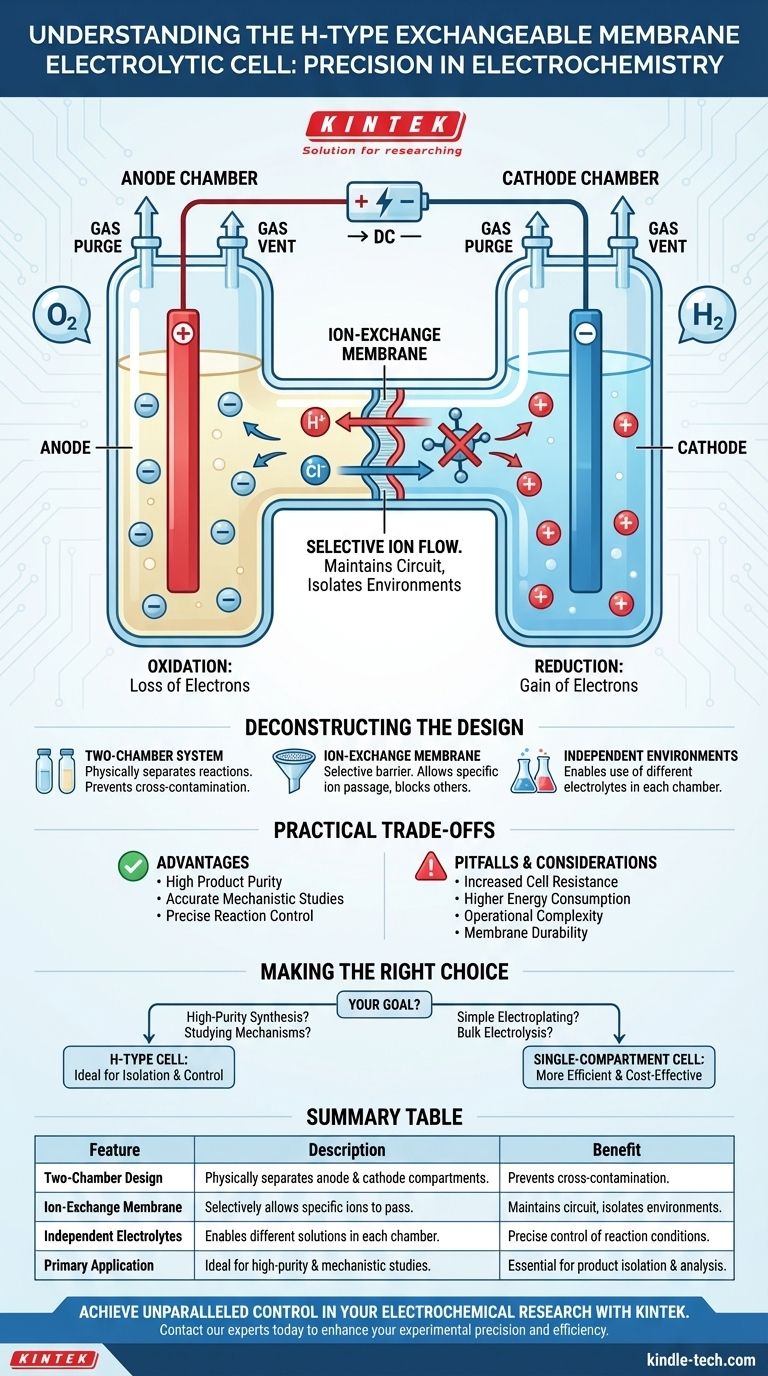
At its core, an H-type exchangeable membrane electrolytic cell is a specialized piece of laboratory equipment designed to physically separate the reactions occurring at the anode and cathode. Its primary function is to use an ion-exchange membrane to create two distinct compartments, allowing specific ions to travel between them while preventing the wholesale mixing of the electrolytes, reactants, and products.
The H-type cell's design is not merely structural; it is functional. It grants researchers precise control over the chemical environment of both the anode and cathode independently, which is essential for studying complex reactions, preventing cross-contamination, and isolating specific products.
Deconstructing the H-Type Cell's Design
The name "H-type" comes from its characteristic shape, which resembles the letter H. This design is fundamental to its function.
The Two-Chamber System
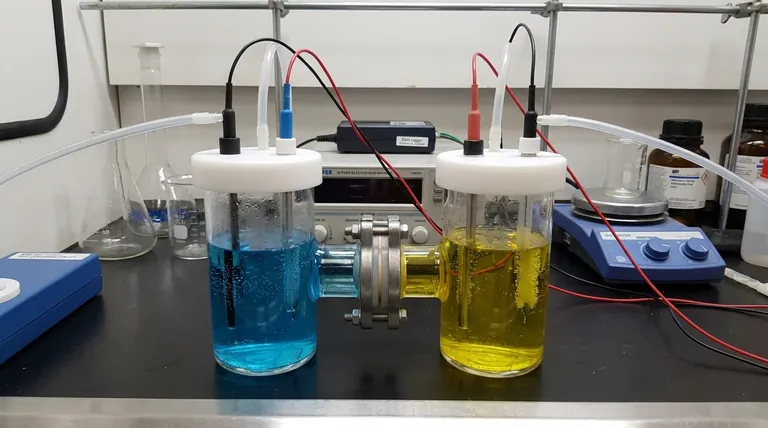
An H-type cell consists of two vertical glass chambers, an anode chamber and a cathode chamber, connected by a horizontal tube. This physical separation is the first step in isolating the two halves of the electrochemical reaction.
The Crucial Role of the Ion-Exchange Membrane
The key component is the ion-exchange membrane situated between the two chambers. This membrane is selectively permeable.
It is designed to allow only certain types of ions (e.g., cations like H⁺ or Na⁺, or anions like Cl⁻) to pass through, effectively completing the electrical circuit. This simultaneously blocks the passage of larger molecules, solvent, and other ions.
Independent Electrode Environments
This separation allows a researcher to use completely different electrolytes in the anode and cathode chambers. This is impossible in a standard single-compartment cell and is the primary reason for using an H-type cell.
Configurable Ports for Electrodes and Gas
Each chamber is equipped with ports to accommodate the necessary components. This typically includes a working electrode, a counter electrode, and a reference electrode, along with smaller ports for purging the solution with gas (like N₂ or O₂) or venting gases produced during the reaction.
The Fundamental Electrochemical Process
The H-type cell operates on the same principles as any electrolytic cell, but with the added layer of control provided by the membrane.
Driving the Reaction
An external power source is applied to the electrodes. This current forces a non-spontaneous chemical reaction to occur.
The Cathode (Reduction)
The cathode is the negative electrode. It attracts positively charged ions (cations) from the electrolyte in its chamber. At the cathode surface, these ions gain electrons in a reduction reaction.
The Anode (Oxidation)
The anode is the positive electrode. It attracts negatively charged ions (anions) from its chamber. At the anode surface, these ions lose electrons in an oxidation reaction.
Ion Flow Through the Membrane
As ions are consumed at the electrodes, a charge imbalance builds up. The ion-exchange membrane allows specific ions to flow from one chamber to the other to neutralize this imbalance and maintain charge neutrality, allowing the reaction to continue.
Understanding the Practical Trade-offs
While powerful, the H-type cell introduces complexities that a researcher must manage.
Advantage: Purity and Control
The most significant advantage is the prevention of cross-contamination. Products made at the anode cannot travel to the cathode and be destroyed, leading to higher product purity and more accurate mechanistic studies.
Pitfall: Increased Cell Resistance
The membrane is a physical barrier that adds electrical resistance to the system. This means a higher voltage is often required to drive the same current compared to a single-compartment cell, which can lead to higher energy consumption.
Pitfall: Operational Complexity
Running an experiment requires careful monitoring. You must observe bubble formation, potential color changes in either electrolyte, and adjust parameters like voltage and current gradually to ensure stable and predictable results.
Consideration: Membrane Choice and Durability
The choice of membrane is critical and depends on the specific ions you need to transport. Membranes can also degrade or become fouled over time, affecting the cell's performance and requiring replacement.
Making the Right Choice for Your Experiment
The decision to use an H-type cell hinges entirely on your experimental goals.
- If your primary focus is synthesizing a high-purity product: The H-cell is ideal, as it prevents the product formed at one electrode from reacting or mixing with reactants at the other.
- If your primary focus is studying complex reaction mechanisms: This cell is essential, as it allows you to isolate and analyze the anolyte and catholyte separately to understand the complete process.
- If your primary focus is simple electroplating or bulk electrolysis where product separation is not critical: A simpler, single-compartment cell may be more efficient and cost-effective due to its lower internal resistance.
Ultimately, the H-type cell empowers precise electrochemical investigation by trading simplicity for unparalleled environmental control.
Summary Table:
| Feature | Description | Benefit |
|---|---|---|
| Two-Chamber Design | Physically separates anode and cathode compartments. | Prevents cross-contamination of reactants and products. |
| Ion-Exchange Membrane | Selectively allows specific ions to pass between chambers. | Maintains electrical circuit while isolating chemical environments. |
| Independent Electrolytes | Enables use of different solutions in each chamber. | Allows for precise, independent control of reaction conditions. |
| Primary Application | Ideal for high-purity synthesis and mechanistic studies. | Essential for experiments requiring product isolation and analysis. |
Ready to achieve unparalleled control in your electrochemical research?
An H-type exchangeable membrane electrolytic cell is essential for experiments demanding high-purity product synthesis and detailed mechanistic studies. KINTEK specializes in providing high-quality lab equipment, including reliable electrolytic cells, to serve the precise needs of research and development laboratories.
Let us help you enhance your experimental precision and efficiency. Contact our experts today to find the perfect electrolytic solution for your laboratory's unique challenges.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- Proton Exchange Membrane for Batteries Lab Applications
People Also Ask
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What are the proper storage conditions for an H-type electrolytic cell? Ensure Long-Term Reliability and Accurate Results
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab



















