To properly prepare an H-type electrolytic cell, you must follow a systematic process that includes meticulous cleaning of all components, a thorough inspection of the cell's integrity, careful preparation of the electrolyte, and activation of the electrodes. These steps are not merely procedural; they are essential for ensuring the safety of the operator and the accuracy and repeatability of the experimental results.
The core principle behind preparing an H-type cell is to establish a pristine and controlled electrochemical environment. Failing to eliminate contaminants or verify component integrity will directly compromise the validity of your data and can introduce significant safety risks.
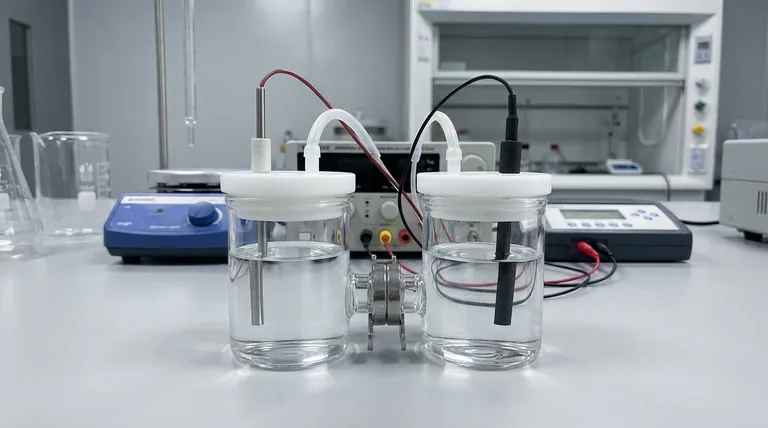
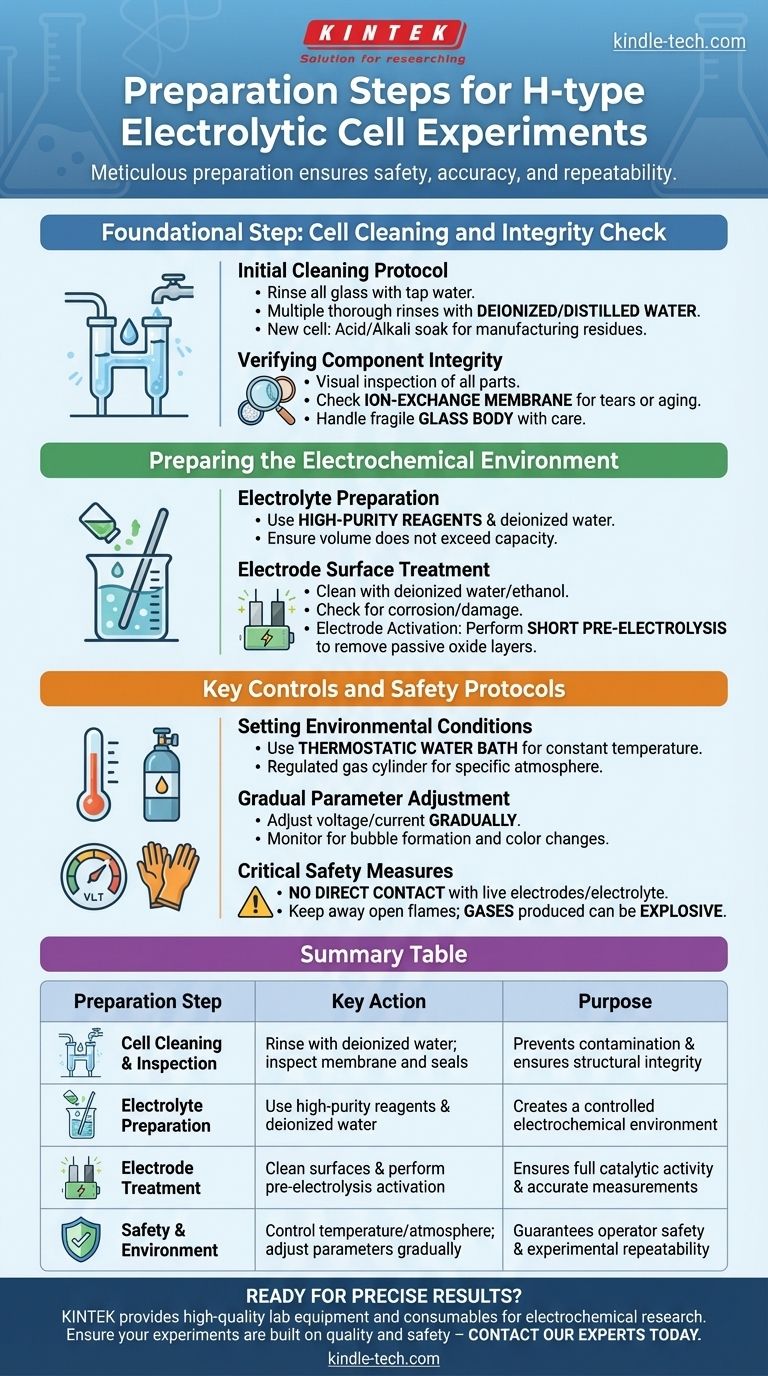
Foundational Step: Cell Cleaning and Integrity Check
Before any chemical or electrical components are introduced, the physical vessel must be verified as clean and structurally sound. This stage prevents cross-contamination and catastrophic failure.
Initial Cleaning Protocol
First, rinse all glass components with tap water to remove loose dust and debris. Follow this with multiple, thorough rinses using deionized or distilled water to remove ionic impurities. For a new cell, a more intensive cleaning, such as soaking in an acid or alkali solution, may be necessary to remove residues from the manufacturing process.
Verifying Component Integrity
A visual inspection is critical. Carefully check that all components are present, including both anode and cathode chambers, the ion-exchange membrane, electrodes, lids, and sealing rings. Pay special attention to the membrane, ensuring it is free from tears, holes, or signs of aging that could lead to electrolyte mixing.
Handling the Glass Body
Remember that the cell body is made of glass and is fragile. It must be handled gently and with care at all times to prevent cracks or breakage, which would render the experiment unsafe and invalid.
Preparing the Electrochemical Environment
With a clean and verified cell, the next phase is to prepare the active chemical and electrical surfaces that will drive the reaction.
Electrolyte Preparation
The electrolyte must be prepared using high-purity chemical reagents and either deionized or distilled water. Using lower-purity materials can introduce unknown ions into your system, creating side reactions and producing inaccurate measurements. Once mixed, pour the electrolyte into the cell, ensuring the volume does not exceed the designated maximum capacity.
Electrode Surface Treatment
The electrode surfaces are the site of your reaction and must be immaculate. Clean the electrodes with deionized water or ethanol to remove any organic or inorganic surface impurities. Check for any signs of corrosion or physical damage; polishing may be required if the surface is compromised.
Electrode Activation
Before beginning data collection, it is crucial to activate the electrodes. This is typically done by running a short pre-electrolysis in the prepared electrolyte. This step removes any passive surface oxide layers that may have formed on the electrodes, ensuring their full catalytic activity is available for the experiment.
Key Controls and Safety Protocols
Proper setup extends beyond the cell itself. You must control the surrounding environment and adhere to strict safety measures throughout the experiment.
Setting Environmental Conditions
Control is the cornerstone of good science. If your experiment requires a constant temperature, use a thermostatic water bath. If it requires a specific atmosphere, such as an inert nitrogen environment, ensure it is properly supplied from a regulated gas cylinder.
Gradual Parameter Adjustment
Once the experiment begins, adjust operating parameters like voltage and current gradually. Monitor the cell for expected activity, such as bubble formation on the electrodes or color changes in the electrolyte. Sudden changes or unexpected phenomena can indicate a problem that needs to be addressed immediately.
Critical Safety Measures
Never make direct physical contact with live electrodes or the electrolyte, as this can lead to severe electric shock or chemical burns. Furthermore, keep all open flames and flammable materials far away from the cell, as gases produced during electrolysis (like hydrogen) can be explosive.
A Checklist for Repeatable Results
Your preparation directly determines the quality of your outcome. Use this guide to align your efforts with your experimental goals.
- If your primary focus is analytical accuracy: The most critical steps are using high-purity reagents for the electrolyte and performing a pre-electrolysis to activate the electrodes.
- If your primary focus is safety and reliability: Prioritize the physical inspection of the ion-exchange membrane and all sealing rings to prevent leaks or cell failure.
- If you are commissioning a new cell: Your most important step is the initial deep cleaning with an acid or alkali soak to remove all manufacturing contaminants.
Thorough preparation is the foundation upon which every successful and reliable electrochemical experiment is built.
Summary Table:
| Preparation Step | Key Action | Purpose |
|---|---|---|
| Cell Cleaning & Inspection | Rinse with deionized water; inspect membrane and seals. | Prevents contamination and ensures structural integrity. |
| Electrolyte Preparation | Use high-purity reagents and deionized water. | Creates a controlled electrochemical environment. |
| Electrode Treatment | Clean surfaces and perform pre-electrolysis activation. | Ensures full catalytic activity and accurate measurements. |
| Safety & Environment | Control temperature/atmosphere; adjust parameters gradually. | Guarantees operator safety and experimental repeatability. |
Ready to achieve precise and reliable results in your lab? Proper preparation is key, and so is having the right equipment. KINTEK specializes in high-quality lab equipment and consumables, including electrolytic cells and pure reagents, designed to meet the rigorous demands of electrochemical research. Ensure your experiments are built on a foundation of quality and safety — contact our experts today to find the perfect solutions for your laboratory needs.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What is the function of an H-type exchangeable membrane electrolytic cell? Master Precise Reaction Control
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide



















