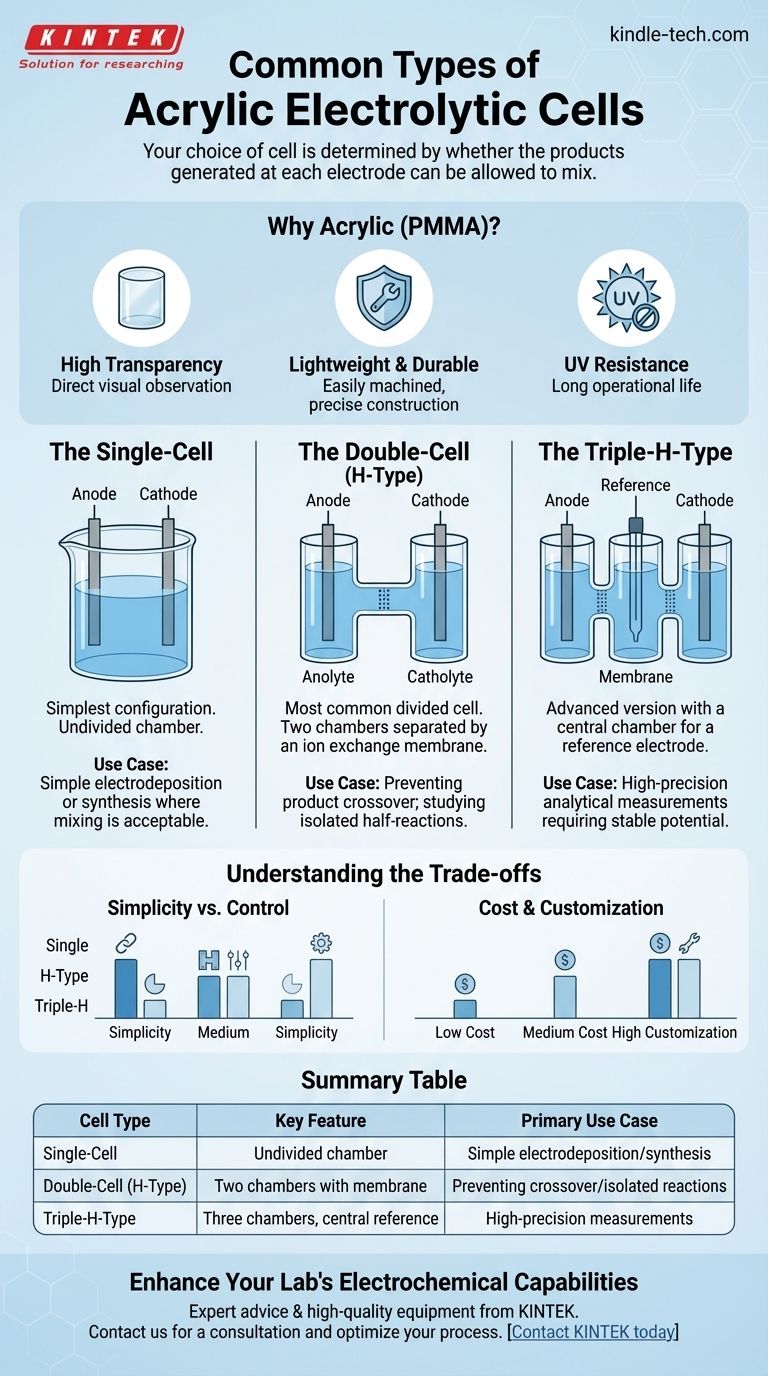
At their core, acrylic electrolytic cells are commonly found in three primary configurations: the single-cell, the double-cell (or H-type), and the triple-H-type. These designs provide standardized, transparent vessels for a wide range of electrochemical experiments, from synthesis to analysis.
The critical difference between cell types is not their complexity, but their ability to separate the chemical reactions occurring at the anode and cathode. Your choice of cell is determined by whether the products generated at each electrode can be allowed to mix.

The Foundation: Why Acrylic?
Before examining the types, it's important to understand why acrylic is the material of choice for these standard laboratory cells.
The Role of PMMA
The cell body is constructed from Polymethyl Methacrylate (PMMA), commonly known as acrylic. This material is chosen for a specific set of highly advantageous properties.
Its high transparency is paramount, allowing for direct visual observation of processes like gas evolution, color changes, or precipitate formation at the electrodes.
Furthermore, PMMA is lightweight, durable, and easily machined, which allows for precise construction and the integration of ports for electrodes, gas lines, and sampling. Its resistance to UV degradation also ensures a long operational life without yellowing.
A Breakdown of Common Cell Architectures
Each cell type is designed to solve a specific experimental problem. The primary distinction is the separation of the compartments containing the anode and cathode.
The Single-Cell
This is the simplest configuration, consisting of a single chamber that holds the electrolyte, anode, and cathode. It is essentially an undivided beaker or tank.
This design is used when the products formed at both electrodes are stable, desired, or do not interfere with each other. It's common for basic electroplating or simple electrolysis where mixing is acceptable.
The Double-Cell (H-Type)
The H-type cell is the most common divided cell. It consists of two separate vertical chambers connected by a horizontal tube or port.
This connecting port is designed to hold an ion exchange membrane or a salt bridge. Its purpose is to physically separate the anolyte (solution around the anode) from the catholyte (solution around thecathode).
This separation is crucial for experiments where the product of one electrode would be consumed or react undesirably at the other electrode. It prevents side reactions and allows for the study of isolated half-reactions.
The Triple-H-Type
The triple-H-type cell is an advanced version of the H-type, introducing a third, central chamber between the anode and cathode compartments.
This central chamber is most often used to house a reference electrode in a controlled environment, completely isolated from the chemical species generated at the working and counter electrodes.
Using this configuration provides an extremely stable potential reference, which is critical for highly sensitive or precise electrochemical measurements like cyclic voltammetry or electrochemical impedance spectroscopy.
Understanding the Trade-offs
Choosing a cell is a balance between experimental needs and practical constraints.
Separation vs. Simplicity
The primary trade-off is simplicity versus control. A single-cell is simple to set up and clean, but offers no control over product mixing. An H-type cell provides critical separation but adds complexity and ionic resistance from the membrane.
Material Compatibility
While PMMA is robust, it is not universally inert. It can be attacked by certain organic solvents. Always verify that your chosen electrolyte and solvent system are compatible with acrylic to prevent cell damage and contamination of your experiment.
Cost and Customization
Single-cells are typically the least expensive. H-type and triple-H-type cells are more complex to manufacture and therefore cost more. However, the ease of processing PMMA means custom designs for specific electrode geometries or port requirements are often feasible.
Making the Right Choice for Your Experiment
Your experimental goal dictates the correct cell architecture.
- If your primary focus is simple electrodeposition or synthesis where products can mix: A single-cell offers the simplest and most cost-effective solution.
- If your primary focus is preventing product crossover or studying isolated electrode reactions: An H-type cell is the standard choice for separating the anolyte and catholyte.
- If your primary focus is high-precision analytical measurement requiring a stable potential: A triple-H-type cell provides the necessary isolation for a reference electrode.
Ultimately, selecting the correct cell architecture is the first step toward generating reliable and meaningful electrochemical data.
Summary Table:
| Cell Type | Key Feature | Primary Use Case |
|---|---|---|
| Single-Cell | Single, undivided chamber | Simple electrodeposition or synthesis where product mixing is acceptable |
| Double-Cell (H-Type) | Two chambers separated by a membrane | Preventing product crossover; studying isolated half-reactions |
| Triple-H-Type | Three chambers with a central reference compartment | High-precision analytical measurements requiring a stable potential reference |
Ready to enhance your lab's electrochemical capabilities?
Choosing the right acrylic electrolytic cell is critical for achieving accurate and reliable results. The expert team at KINTEK specializes in providing high-quality lab equipment, including a full range of acrylic electrolytic cells and consumables tailored to your specific research needs.
We understand the nuances of electrochemical experiments and can help you select the perfect cell design—whether you need the simplicity of a single-cell or the precision of a triple-H-type configuration.
Let us help you optimize your process. Contact KINTEK today for a consultation and discover the difference the right equipment can make.
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- How should failures or malfunctions of an H-type electrolytic cell be handled? A Guide to Safe and Effective Troubleshooting
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results



















