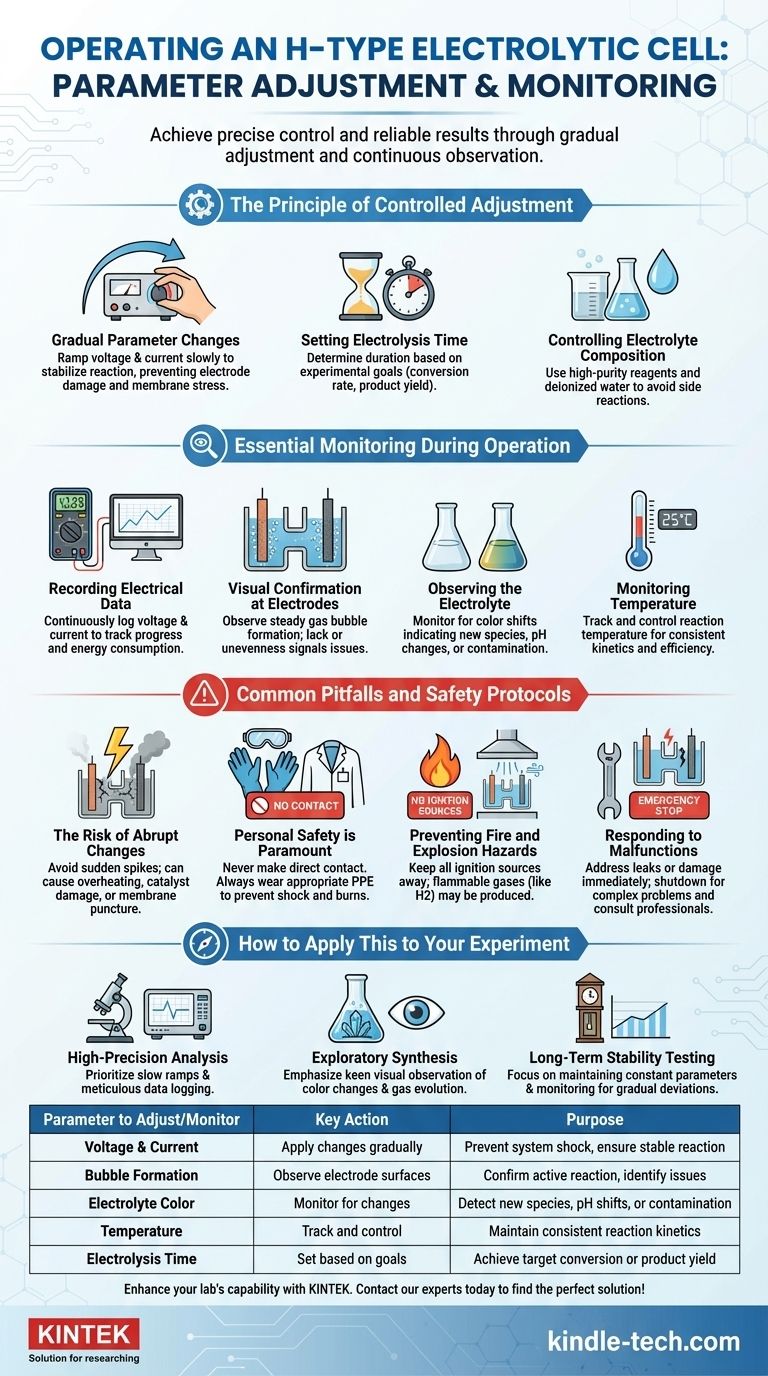
To properly operate an H-type electrolytic cell, you must adjust experimental parameters like voltage and current gradually rather than abruptly. Concurrently, you need to vigilantly monitor the physical state of the cell by observing bubble formation on the electrode surfaces and any color changes in the electrolyte to ensure the reaction is proceeding as expected and to identify any issues immediately.
The key to successful and repeatable electrolysis is not just setting parameters, but actively managing a dynamic system. This requires combining slow, controlled adjustments with continuous observation of both electrical data and physical indicators to maintain precise control and ensure safety.

The Principle of Controlled Adjustment
To achieve a stable and predictable electrochemical reaction, your inputs must be deliberate and measured. Abrupt changes can shock the system, leading to inconsistent results and potential damage.
Gradual Parameter Changes
Instead of jumping to a target voltage or current, ramp it up slowly. This allows the system to stabilize at each step, ensuring a more uniform reaction and preventing potential damage to the electrode surface or the ion-exchange membrane.
Setting Electrolysis Time
The duration of the experiment is a critical parameter that you control directly. This should be determined by your experimental goals, such as achieving a specific conversion rate or producing a target amount of product.
Controlling Electrolyte Composition
While typically set during preparation, the composition and concentration of the electrolyte are key variables. Ensuring high-purity reagents and deionized water is crucial to prevent side reactions from impurities.
Essential Monitoring During Operation
Effective monitoring is a multi-faceted process. You must track both the quantitative electrical data and the qualitative physical changes occurring within the cell.
Recording Electrical Data
Your primary source of quantitative information is your power supply and detection instruments. Continuously monitor and record the voltage and current to track the reaction's progress and energy consumption.
Visual Confirmation at the Electrodes
Observe the surface of both the anode and cathode. The steady formation of gas bubbles is a primary indicator that the electrochemical reaction is active. A lack of bubbles or uneven formation can signal a problem with the electrode or circuit.
Observing the Electrolyte
Pay close attention to the electrolyte in both chambers. A change in color can indicate the formation of a new chemical species, a shift in pH, or a potential contamination issue.
Monitoring Temperature
Reaction conditions like temperature must be controlled. Any significant deviation can alter reaction kinetics and efficiency, so it is a crucial parameter to monitor, especially during long experiments.
Common Pitfalls and Safety Protocols
Understanding the risks associated with electrolysis is fundamental to safe and effective operation. Inattention can lead to failed experiments, equipment damage, or personal injury.
The Risk of Abrupt Changes
Sudden spikes in voltage or current can cause localized overheating, damage the electrode catalyst layer, or even puncture the delicate ion-exchange membrane, compromising the entire experiment.
Personal Safety is Paramount
Never make direct contact with active electrodes or the electrolyte. This poses a significant risk of electric shock and chemical burns. Always wear appropriate personal protective equipment (PPE).
Preventing Fire and Explosion Hazards
Electrolysis can produce flammable gases, such as hydrogen. It is imperative to keep all open flames, sparks, and other ignition sources far away from the experimental setup to prevent fire or explosion.
Responding to Malfunctions
If you observe electrolyte leakage or suspect electrode damage, address the issue immediately. Simple faults may be correctable, but complex problems require shutting down the system and consulting with professional repair personnel.
How to Apply This to Your Experiment
Your specific monitoring and adjustment strategy should align with your primary experimental goal.
- If your primary focus is high-precision quantitative analysis: Prioritize extremely slow parameter ramps and meticulous logging of all electrical data.
- If your primary focus is exploratory synthesis: Emphasize keen visual observation of color changes and gas evolution, as these are often the first indicators of a new or unexpected reaction.
- If your primary focus is long-term stability testing: Focus on maintaining constant parameters and monitoring for any gradual deviation in voltage, current, or physical appearance over time.
Ultimately, mastering the operation of an electrolytic cell comes from treating it as a responsive system that requires both precise control and careful observation.
Summary Table:
| Parameter to Adjust/Monitor | Key Action | Purpose |
|---|---|---|
| Voltage & Current | Apply changes gradually | Prevent system shock, ensure stable reaction |
| Bubble Formation | Observe electrode surfaces | Confirm active reaction, identify issues |
| Electrolyte Color | Monitor for changes | Detect new species, pH shifts, or contamination |
| Temperature | Track and control | Maintain consistent reaction kinetics |
| Electrolysis Time | Set based on goals | Achieve target conversion or product yield |
Achieve precise control and reliable results in your electrochemical experiments with KINTEK. Our specialized lab equipment, including reliable electrolytic cells and power supplies, is designed to support the meticulous parameter adjustment and monitoring your research demands. Enhance your lab's capability for high-precision analysis, exploratory synthesis, or long-term stability testing. Contact our experts today to find the perfect solution for your laboratory's needs!
Visual Guide
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
People Also Ask
- What experimental conditions need to be controlled when using an H-type electrolytic cell? Ensure Reliable and Repeatable Results
- What preparation steps are needed before starting an experiment with an H-type electrolytic cell? A Guide to Safe and Accurate Results
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the key safety operation guidelines for using the H-type electrolytic cell? Best Practices for Your Lab
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide



















